Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening With a Blood Test That Meets the Centers for Medicare & Medicaid Services Coverage Decision
- PMID: 38552671
- PMCID: PMC11193618
- DOI: 10.1053/j.gastro.2024.02.012
Effectiveness and Cost-Effectiveness of Colorectal Cancer Screening With a Blood Test That Meets the Centers for Medicare & Medicaid Services Coverage Decision
Abstract
Background & aims: A blood-based colorectal cancer (CRC) screening test may increase screening participation. However, blood tests may be less effective than current guideline-endorsed options. The Centers for Medicare & Medicaid Services (CMS) covers blood tests with sensitivity of at least 74% for detection of CRC and specificity of at least 90%. In this study, we investigate whether a blood test that meets these criteria is cost-effective.
Methods: Three microsimulation models for CRC (MISCAN-Colon, CRC-SPIN, and SimCRC) were used to estimate the effectiveness and cost-effectiveness of triennial blood-based screening (from ages 45 to 75 years) compared to no screening, annual fecal immunochemical testing (FIT), triennial stool DNA testing combined with an FIT assay, and colonoscopy screening every 10 years. The CMS coverage criteria were used as performance characteristics of the hypothetical blood test. We varied screening ages, test performance characteristics, and screening uptake in a sensitivity analysis.
Results: Without screening, the models predicted 77-88 CRC cases and 32-36 CRC deaths per 1000 individuals, costing $5.3-$5.8 million. Compared to no screening, blood-based screening was cost-effective, with an additional cost of $25,600-$43,700 per quality-adjusted life-year gained (QALYG). However, compared to FIT, triennial stool DNA testing combined with FIT, and colonoscopy, blood-based screening was not cost-effective, with both a decrease in QALYG and an increase in costs. FIT remained more effective (+5-24 QALYG) and less costly (-$3.2 to -$3.5 million) than blood-based screening even when uptake of blood-based screening was 20 percentage points higher than uptake of FIT.
Conclusion: Even with higher screening uptake, triennial blood-based screening, with the CMS-specified minimum performance sensitivity of 74% and specificity of 90%, was not projected to be cost-effective compared with established strategies for colorectal cancer screening.
Keywords: Biomarkers; Colorectal Cancer; Cost-Effectiveness.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors have no conflict of interest to disclose.
Figures
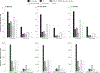
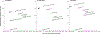
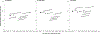
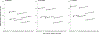
References
-
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: a cancer journal for clinicians 2023; 73(1):17–48. - PubMed
-
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: a cancer journal for clinicians 2018; 68(4):250–81. - PubMed
-
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. Jama 2016; 315(23):2576–94. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

