Protein kinase A is a functional component of focal adhesions
- PMID: 38552737
- PMCID: PMC11044056
- DOI: 10.1016/j.jbc.2024.107234
Protein kinase A is a functional component of focal adhesions
Abstract
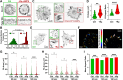
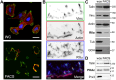
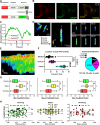
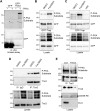
Focal adhesions (FAs) form the junction between extracellular matrix (ECM)-bound integrins and the actin cytoskeleton and also transmit signals that regulate cell adhesion, cytoskeletal dynamics, and cell migration. While many of these signals are rooted in reversible tyrosine phosphorylation, phosphorylation of FA proteins on Ser/Thr residues is far more abundant yet its mechanisms and consequences are far less understood. The cAMP-dependent protein kinase (protein kinase A; PKA) has important roles in cell adhesion and cell migration and is both an effector and regulator of integrin-mediated adhesion to the ECM. Importantly, subcellular localization plays a critically important role in specifying PKA function. Here, we show that PKA is present in isolated FA-cytoskeleton complexes and active within FAs in live cells. Furthermore, using kinase-catalyzed biotinylation of isolated FA-cytoskeleton complexes, we identify 53 high-stringency candidate PKA substrates within FAs. From this list, we validate tensin-3 (Tns3)-a well-established molecular scaffold, regulator of cell migration, and a component of focal and fibrillar adhesions-as a novel direct substrate for PKA. These observations identify a new pathway for phospho-regulation of Tns3 and, importantly, establish a new and important niche for localized PKA signaling and thus provide a foundation for further investigation of the role of PKA in the regulation of FA dynamics and signaling.
Keywords: biosensor; focal adhesions; phosphorylation; protein kinase A (PKA); signal transduction.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Update of
-
Protein Kinase A is a Functional Component of Focal Adhesions.bioRxiv [Preprint]. 2024 Mar 2:2023.08.18.553932. doi: 10.1101/2023.08.18.553932. bioRxiv. 2024. Update in: J Biol Chem. 2024 May;300(5):107234. doi: 10.1016/j.jbc.2024.107234. PMID: 37645771 Free PMC article. Updated. Preprint.
References
-
- Jockusch B.M., Bubeck P., Giehl K., Kroemker M., Moschner J., Rothkegel M., et al. The molecular architecture of focal adhesions. Annu. Rev. Cell Dev. Biol. 1995;11:379–416. - PubMed
-
- Dubash A.D., Menold M.M., Samson T., Boulter E., Garcia-Mata R., Doughman R., et al. Chapter 1. Focal adhesions: new angles on an old structure. Int. Rev. Cell Mol. Biol. 2009;277:1–65. - PubMed
-
- Humphries J.D., Chastney M.R., Askari J.A., Humphries M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2019;56:14–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

