Acute and two-week effects of neotame, stevia rebaudioside M and sucrose-sweetened biscuits on postprandial appetite and endocrine response in adults with overweight/obesity-a randomised crossover trial from the SWEET consortium
- PMID: 38553262
- PMCID: PMC11026940
- DOI: 10.1016/j.ebiom.2024.105005
Acute and two-week effects of neotame, stevia rebaudioside M and sucrose-sweetened biscuits on postprandial appetite and endocrine response in adults with overweight/obesity-a randomised crossover trial from the SWEET consortium
Abstract
Background: Sweeteners and sweetness enhancers (S&SE) are used to replace energy yielding sugars and maintain sweet taste in a wide range of products, but controversy exists about their effects on appetite and endocrine responses in reduced or no added sugar solid foods. The aim of the current study was to evaluate the acute (1 day) and repeated (two-week daily) ingestive effects of 2 S&SE vs. sucrose formulations of biscuit with fruit filling on appetite and endocrine responses in adults with overweight and obesity.
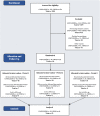
Methods: In a randomised crossover trial, 53 healthy adults (33 female, 20 male) with overweight/obesity in England and France consumed biscuits with fruit filling containing 1) sucrose, or reformulated with either 2) Stevia Rebaudioside M (StRebM) or 3) Neotame daily during three, two-week intervention periods with a two-week washout. The primary outcome was composite appetite score defined as [desire to eat + hunger + (100 - fullness) + prospective consumption]/4.
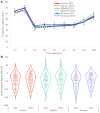
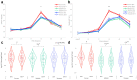
Findings: Each formulation elicited a similar reduction in appetite sensations (3-h postprandial net iAUC). Postprandial insulin (2-h iAUC) was lower after Neotame (95% CI (0.093, 0.166); p < 0.001; d = -0.71) and StRebM (95% CI (0.133, 0.205); p < 0.001; d = -1.01) compared to sucrose, and glucose was lower after StRebM (95% CI (0.023, 0.171); p < 0.05; d = -0.39) but not after Neotame (95% CI (-0.007, 0.145); p = 0.074; d = -0.25) compared to sucrose. There were no differences between S&SE or sucrose formulations on ghrelin, glucagon-like peptide 1 or pancreatic polypeptide iAUCs. No clinically meaningful differences between acute vs. two-weeks of daily consumption were found.
Interpretation: In conclusion, biscuits reformulated to replace sugar using StRebM or Neotame showed no differences in appetite or endocrine responses, acutely or after a two-week exposure, but can reduce postprandial insulin and glucose response in adults with overweight or obesity.
Funding: The present study was funded by the Horizon 2020 program: Sweeteners and sweetness enhancers: Impact on health, obesity, safety and sustainability (acronym: SWEET, grant no: 774293).
Keywords: Appetite; Endocrine response; Glycaemia; Solid food; Sweeteners; Sweetness enhancers.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests JCGH and JAH are in receipt of research funding from the American Beverage Association. ARA has received honoraria from Nestle, Unilever and the International Sweeteners Association. University of Liverpool has received income from International Food Information Council by CH. CH has received honoraria for work with Food Standards Agency Advisory Committee on Social Sciences. MW was previously contracted to research funded by AstraZeneca through funding paid to University of Liverpool. CS is a paid employee of Cargill, Inc. The University of Leeds has received income from consultancy for Mars Inc by JCGH. EAR has received honoraria for manuscript writing from Institute of Life Sciences (ILSI-Europe).
Figures


References
-
- Guideline: sugars intake for adults and children. World Health Organization; 2015. - PubMed
-
- San-Cristobal R., Navas-Carretero S., Martinez-Gonzalez M.A., Ordovas J.M., Martinez J.A. Contribution of macronutrients to obesity: implications for precision nutrition. Nat Rev Endocrinol. 2020;16(6):305–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

