Structure of alpha-synuclein fibrils derived from human Lewy body dementia tissue
- PMID: 38553463
- PMCID: PMC10980826
- DOI: 10.1038/s41467-024-46832-5
Structure of alpha-synuclein fibrils derived from human Lewy body dementia tissue
Abstract
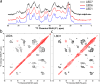
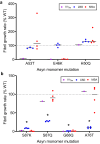
The defining feature of Parkinson disease (PD) and Lewy body dementia (LBD) is the accumulation of alpha-synuclein (Asyn) fibrils in Lewy bodies and Lewy neurites. Here we develop and validate a method to amplify Asyn fibrils extracted from LBD postmortem tissue samples and use solid state nuclear magnetic resonance (SSNMR) studies to determine atomic resolution structure. Amplified LBD Asyn fibrils comprise a mixture of single protofilament and two protofilament fibrils with very low twist. The protofilament fold is highly similar to the fold determined by a recent cryo-electron microscopy study for a minority population of twisted single protofilament fibrils extracted from LBD tissue. These results expand the structural characterization of LBD Asyn fibrils and approaches for studying disease mechanisms, imaging agents and therapeutics targeting Asyn.
© 2024. The Author(s).
Conflict of interest statement
Authors specified below have a patent application pending titled “Tissue-Seeded Fibrils and Methods of Making and Using Same”. Patent applicant: Washington University in St. Louis, Name of inventors: Paul T. Kotzbauer, Dhruva D. Dhavale, Rebecca Miller and Jennifer Y. O’Shea, Application number: 17/858817, Status of application: Pending. The patent application covers the process of generating amplified fibrils, its methods and composition. Other authors declares no competing interests.
Figures







Update of
-
Structure of alpha-synuclein fibrils derived from human Lewy body dementia tissue.bioRxiv [Preprint]. 2023 Jan 10:2023.01.09.523303. doi: 10.1101/2023.01.09.523303. bioRxiv. 2023. Update in: Nat Commun. 2024 Mar 29;15(1):2750. doi: 10.1038/s41467-024-46832-5. PMID: 36711931 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

