Predictors of nirmatrelvir-ritonavir receipt among COVID-19 patients in a large US health system
- PMID: 38553527
- PMCID: PMC10980791
- DOI: 10.1038/s41598-024-57633-7
Predictors of nirmatrelvir-ritonavir receipt among COVID-19 patients in a large US health system
Abstract
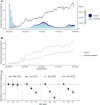
A clear understanding of real-world uptake of nirmatrelvir-ritonavir for treatment of SARS-CoV-2 can inform treatment allocation strategies and improve interpretation of effectiveness studies. We used data from a large US healthcare system to describe nirmatrelvir-ritonavir dispenses among all SARS-CoV-2 positive patients aged ≥ 12 years meeting recommended National Institutes of Health treatment eligibility criteria for the study period between 1 January and 31 December, 2022. Overall, 10.9% (N = 34,791/319,900) of treatment eligible patients with SARS-CoV-2 infections received nirmatrelvir-ritonavir over the study period. Although uptake of nirmatrelvir-ritonavir increased over time, by the end of 2022, less than a quarter of treatment eligible patients with SARS-CoV-2 infections had received nirmatrelvir-ritonavir. Across patient demographics, treatment was generally consistent with tiered treatment guidelines, with dispenses concentrated among patients aged ≥ 65 years (14,706/63,921; 23.0%), and with multiple comorbidities (10,989/54,431; 20.1%). However, neighborhoods of lower socioeconomic status (upper third of neighborhood deprivation index [NDI]) had between 12% (95% CI: 7-18%) and 28% (25-32%) lower odds of treatment dispense over the time periods studied compared to the lower third of NDI distribution, even after accounting for demographic and clinical characteristics. A limited chart review (N = 40) confirmed that in some cases a decision not to treat was appropriate and aligned with national guidelines to use clinical judgement on a case-by-case basis. There is a need to enhance patient and provider awareness on the availability and benefits of nirmatrelvir-ritonavir for the treatment of COVID-19 illness.
© 2024. The Author(s).
Conflict of interest statement
JAL has received grants and consultancy fees from Pfizer. SYT and TF have received grants from Pfizer paid directly to their institution. LP and JMM are employees of Pfizer and hold stock and stock options in Pfizer. BKA receives research support from Dynavax, Moderna, GlaxoSmithKline, Pfizer and Genentech, for projects outside of the submitted work. JS has received grants from Pfizer, ALK Inc., Novavax and Dynavax Technologies paid directly to his institution. DM, VH and JK have no conflicts of interest to declare. This study was funded by Pfizer.
Figures
Similar articles
-
COVID-19 hospitalization risk after outpatient nirmatrelvir/ritonavir use, January to August 2022, North Carolina.J Antimicrob Chemother. 2024 Apr 2;79(4):859-867. doi: 10.1093/jac/dkae042. J Antimicrob Chemother. 2024. PMID: 38380946 Free PMC article.
-
Real-world cost-effectiveness of nirmatrelvir-ritonavir as treatment for SARS-CoV-2 infection in the Belgian setting with omicron variant.Front Public Health. 2025 Feb 3;12:1432821. doi: 10.3389/fpubh.2024.1432821. eCollection 2024. Front Public Health. 2025. PMID: 39963121 Free PMC article.
-
Nirmatrelvir/ritonavir use in pregnant women with SARS-CoV-2 Omicron infection: a target trial emulation.Nat Med. 2024 Jan;30(1):112-116. doi: 10.1038/s41591-023-02674-0. Epub 2023 Nov 1. Nat Med. 2024. PMID: 37913816 Clinical Trial.
-
A Comprehensive Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Drug Interactions of Nirmatrelvir/Ritonavir.Clin Pharmacokinet. 2024 Jan;63(1):27-42. doi: 10.1007/s40262-023-01339-y. Epub 2024 Jan 4. Clin Pharmacokinet. 2024. PMID: 38177893 Free PMC article. Review.
-
[Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination].Rev Esp Quimioter. 2022 Jun;35(3):236-240. doi: 10.37201/req/002.2022. Epub 2022 Feb 21. Rev Esp Quimioter. 2022. PMID: 35183067 Free PMC article. Review. Spanish.
Cited by
-
Factors Associated with Seeking and Receiving Home Antiviral Therapy During the COVID-19 Pandemic.medRxiv [Preprint]. 2025 May 28:2025.05.25.25328310. doi: 10.1101/2025.05.25.25328310. medRxiv. 2025. PMID: 40492088 Free PMC article. Preprint.
-
Association between nirmatrelvir/ritonavir treatment and antibiotic prescribing in the outpatient setting among patients with COVID-19.Microbiol Spectr. 2025 Apr;13(4):e0320924. doi: 10.1128/spectrum.03209-24. Epub 2025 Mar 5. Microbiol Spectr. 2025. PMID: 40042306 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

