Control of breathing by orexinergic signaling in the nucleus tractus solitarii
- PMID: 38553555
- PMCID: PMC10980752
- DOI: 10.1038/s41598-024-58075-x
Control of breathing by orexinergic signaling in the nucleus tractus solitarii
Abstract
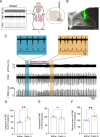
Orexin signaling plays a facilitatory role in respiration. Abnormalities in orexin levels correlate with disordered breathing patterns and impaired central respiratory chemoreception. Nucleus tractus solitarii (NTS) neurons expressing the transcription factor Phox2b contribute to the chemoreceptive regulation of respiration. However, the extent to which orexinergic signaling modulates respiratory activity in these Phox2b-expressing NTS neurons remains unclear. In the present study, the injection of orexin A into the NTS significantly increased the firing rate of the phrenic nerve. Further analysis using fluorescence in situ hybridization and immunohistochemistry revealed that orexin 1 receptors (OX1Rs) were primarily located in the ventrolateral subdivision of the NTS and expressed in 25% of Phox2b-expressing neurons. Additionally, electrophysiological recordings showed that exposure to orexin A increased the spontaneous firing rate of Phox2b-expressing neurons. Immunostaining experiments with cFos revealed that the OX1R-residing Phox2b-expressing neurons were activated by an 8% CO2 stimulus. Crucially, OX1R knockdown in these NTS neurons notably blunted the ventilatory response to 8% CO2, alongside an increase in sigh-related apneas. In conclusion, orexinergic signaling in the NTS facilitates breathing through the activation of OX1Rs, which induces the depolarization of Phox2b-expressing neurons. OX1Rs are essential for the involvement of Phox2b-expressing NTS neurons in the hypercapnic ventilatory response.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

