Day length regulates gonadotrope proliferation and reproduction via an intra-pituitary pathway in the model vertebrate Oryzias latipes
- PMID: 38553567
- PMCID: PMC10980775
- DOI: 10.1038/s42003-024-06059-y
Day length regulates gonadotrope proliferation and reproduction via an intra-pituitary pathway in the model vertebrate Oryzias latipes
Abstract
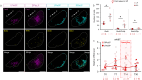
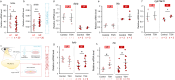
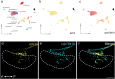
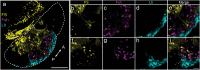
In seasonally breeding mammals and birds, the production of the hormones that regulate reproduction (gonadotropins) is controlled by a complex pituitary-brain-pituitary pathway. Indeed, the pituitary thyroid-stimulating hormone (TSH) regulates gonadotropin expression in pituitary gonadotropes, via dio2-expressing tanycytes, hypothalamic Kisspeptin, RFamide-related peptide, and gonadotropin-releasing hormone neurons. However, in fish, how seasonal environmental signals influence gonadotropins remains unclear. In addition, the seasonal regulation of gonadotrope (gonadotropin-producing cell) proliferation in the pituitary is, to the best of our knowledge, not elucidated in any vertebrate group. Here, we show that in the vertebrate model Japanese medaka (Oryzias latipes), a long day seasonally breeding fish, photoperiod (daylength) not only regulates hormone production by the gonadotropes but also their proliferation. We also reveal an intra-pituitary pathway that regulates gonadotrope cell number and hormone production. In this pathway, Tsh regulates gonadotropes via folliculostellate cells within the pituitary. This study suggests the existence of an alternative regulatory mechanism of seasonal gonadotropin production in fish.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Migaud H, Davie A, Taylor JF. Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J. Fish. Biol. 2010;76:27–68. - PubMed
-
- Kanda S. Evolution of the regulatory mechanisms for the hypothalamic-pituitary-gonadal axis in vertebrates–hypothesis from a comparative view. Gen. Comp. Endocrinol. 2019;284:113075. - PubMed
-
- Yaron, Z. & Levavi-Sivan, B. in Encyclopedia of Fish Physiology Vol. 2 (ed A. P. Farrell) 1500–1508 (Academic Press, 2011). 10.1016/B978-0-12-374553-8.00058-7.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

