Cryo-EM structures of amyloid-β and tau filaments in Down syndrome
- PMID: 38553642
- PMCID: PMC11189299
- DOI: 10.1038/s41594-024-01252-3
Cryo-EM structures of amyloid-β and tau filaments in Down syndrome
Abstract
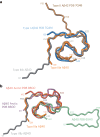
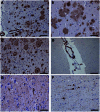
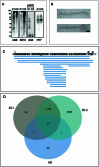
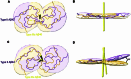
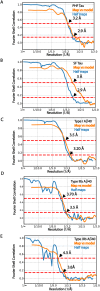
Adult individuals with Down syndrome (DS) develop Alzheimer disease (AD). Whether there is a difference between AD in DS and AD regarding the structure of amyloid-β (Aβ) and tau filaments is unknown. Here we report the structure of Aβ and tau filaments from two DS brains. We found two Aβ40 filaments (types IIIa and IIIb) that differ from those previously reported in sporadic AD and two types of Aβ42 filaments (I and II) identical to those found in sporadic and familial AD. Tau filaments (paired helical filaments and straight filaments) were identical to those in AD, supporting the notion of a common mechanism through which amyloids trigger aggregation of tau. This knowledge is important for understanding AD in DS and assessing whether adults with DS could be included in AD clinical trials.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






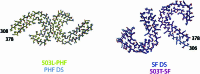

Similar articles
-
Cryo-EM structures reveal tau filaments from Down syndrome adopt Alzheimer's disease fold.Acta Neuropathol Commun. 2024 Jun 12;12(1):94. doi: 10.1186/s40478-024-01806-y. Acta Neuropathol Commun. 2024. PMID: 38867338 Free PMC article.
-
Tau filaments from multiple cases of sporadic and inherited Alzheimer's disease adopt a common fold.Acta Neuropathol. 2018 Nov;136(5):699-708. doi: 10.1007/s00401-018-1914-z. Epub 2018 Oct 1. Acta Neuropathol. 2018. PMID: 30276465 Free PMC article.
-
Cryo-EM structures of tau filaments from Alzheimer's disease.Nature. 2017 Jul 13;547(7662):185-190. doi: 10.1038/nature23002. Epub 2017 Jul 5. Nature. 2017. PMID: 28678775 Free PMC article.
-
Role of tau protein in neuronal damage in Alzheimer's disease and Down syndrome.Arch Med Res. 2012 Nov;43(8):645-54. doi: 10.1016/j.arcmed.2012.10.012. Epub 2012 Nov 7. Arch Med Res. 2012. PMID: 23142525 Review.
-
Key Peptides and Proteins in Alzheimer's Disease.Curr Protein Pept Sci. 2019;20(6):577-599. doi: 10.2174/1389203720666190103123434. Curr Protein Pept Sci. 2019. PMID: 30605056 Review.
Cited by
-
Membrane-assisted Aβ40 aggregation pathways.bioRxiv [Preprint]. 2024 Sep 8:2024.09.05.611426. doi: 10.1101/2024.09.05.611426. bioRxiv. 2024. Update in: Cell Rep Phys Sci. 2025 Feb 19;6(2):102436. doi: 10.1016/j.xcrp.2025.102436. PMID: 39282376 Free PMC article. Updated. Preprint.
-
Cryo-EM structures reveal tau filaments from Down syndrome adopt Alzheimer's disease fold.Acta Neuropathol Commun. 2024 Jun 12;12(1):94. doi: 10.1186/s40478-024-01806-y. Acta Neuropathol Commun. 2024. PMID: 38867338 Free PMC article.
-
In Vivo Seeding of Amyloid-β Protein and Implications in Modeling Alzheimer's Disease Pathology.Biomolecules. 2025 Apr 11;15(4):571. doi: 10.3390/biom15040571. Biomolecules. 2025. PMID: 40305318 Free PMC article. Review.
-
Membrane-assisted Aβ40 aggregation pathways.Cell Rep Phys Sci. 2025 Feb 19;6(2):102436. doi: 10.1016/j.xcrp.2025.102436. Epub 2025 Feb 11. Cell Rep Phys Sci. 2025. PMID: 40083905 Free PMC article.
-
Lecanemab Binds to Transgenic Mouse Model-Derived Amyloid-β Fibril Structures Resembling Alzheimer's Disease Type I, Type II and Arctic Folds.Neuropathol Appl Neurobiol. 2025 Jun;51(3):e70022. doi: 10.1111/nan.70022. Neuropathol Appl Neurobiol. 2025. PMID: 40495448 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

