Effects of mifepristone on adipocyte differentiation in mouse 3T3-L1 cells
- PMID: 38553665
- PMCID: PMC10981365
- DOI: 10.1186/s11658-024-00559-9
Effects of mifepristone on adipocyte differentiation in mouse 3T3-L1 cells
Abstract
Background: Both glucocorticoid receptor and peroxisome proliferator-activated receptor-γ (PPARγ) play a critical role in adipocyte differentiation. Mifepristone is not only an antagonist of the glucocorticoid receptor but also an agonist of PPARγ. Therefore, the present study investigated the effect of mifepristone on adipocyte differentiation.
Methods: Mouse 3T3-L1 cells were used as a model for adipocyte differentiation. The lipid droplet formation was evaluated with Bodipy493/503 staining and the expression of adipocyte markers [adiponectin and adipocyte fatty acid binding protein-4 (Fabp4)] was evaluated with quantitative PCR and immunoblot analyses for indication of adipocyte differentiation. siRNA and neutralizing antibodies were used to elucidate the molecular mechanism of mifepristone-induced adipocyte differentiation. Luciferase reporter assay was used to examine the effect of mifepristone on the promoter activity of PPAR-response element (PPRE). The DNA microarray analysis was used to characterize the transcriptome of the mifepristone-induced adipocytes. In vivo adipogenic effect of mifepristone was examined in mice.
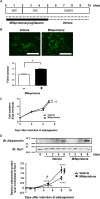
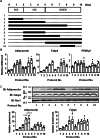
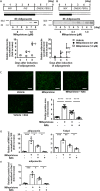
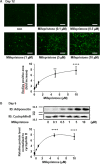
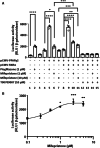
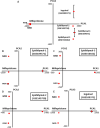
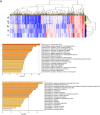
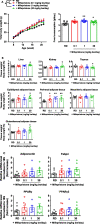
Results: Mifepristone not only enhanced adipocyte differentiation induced by the conventional protocol consisting of insulin, dexamethasone and 3-isobutyl-1-methylxanthine but also induced adipocyte differentiation alone, as evidenced by lipid droplets formation and induction of the expression of adiponectin and Fabp4. These effects were inhibited by an adiponectin-neutralizing antibody and a PPARγ antagonist. Mifepristone activated the promoter activity of PPRE in a manner sensitive to PPARγ antagonist. A principal component analysis (PCA) of DNA microarray data revealed that the mifepristone-induced adipocytes represent some characteristics of the in situ adipocytes in normal adipose tissues to a greater extent than those induced by the conventional protocol. Mifepristone administration induced an increase in the weight of epididymal, perirenal and gluteofemoral adipose tissues.
Conclusions: Mifepristone alone is capable of inducing adipocyte differentiation in 3T3-L1 cells and adipogenesis in vivo. PPARγ plays a critical role in the mifepristone-induced adipocyte differentiation. Mifepristone-induced adipocytes are closer to the in situ adipocytes than those induced by the conventional protocol. The present study proposes a single treatment with mifepristone as a novel protocol to induce more physiologically relevant adipocytes in 3T3-L1 cells than the conventional protocol.
Keywords: Adipocyte differentiation; Mifepristone; Neutralizing antibodies; PPARγ; siRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests in association with the present study.
Figures




Similar articles
-
Identification of DDX5 as an indispensable activator of the glucocorticoid receptor in adipocyte differentiation.FEBS J. 2023 Feb;290(4):988-1007. doi: 10.1111/febs.16618. Epub 2022 Sep 27. FEBS J. 2023. PMID: 36071319
-
Coprinus comatus cap inhibits adipocyte differentiation via regulation of PPARγ and Akt signaling pathway.PLoS One. 2014 Sep 2;9(9):e105809. doi: 10.1371/journal.pone.0105809. eCollection 2014. PLoS One. 2014. PMID: 25181477 Free PMC article.
-
Torreya nucifera seed oil improves 3T3-L1 adipocyte differentiation.BMC Complement Med Ther. 2021 Oct 7;21(1):255. doi: 10.1186/s12906-021-03429-5. BMC Complement Med Ther. 2021. PMID: 34620154 Free PMC article.
-
Small heterodimer partner-interacting leucine zipper protein inhibits adipogenesis by regulating peroxisome proliferator-activated receptor γ activity.Life Sci. 2015 Jul 1;132:49-54. doi: 10.1016/j.lfs.2015.03.021. Epub 2015 Apr 18. Life Sci. 2015. PMID: 25896661
-
Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds.Mol Biol Rep. 2021 Jan;48(1):743-761. doi: 10.1007/s11033-020-06036-8. Epub 2020 Dec 4. Mol Biol Rep. 2021. PMID: 33275195 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

