Mice lacking DIO3 exhibit sex-specific alterations in circadian patterns of corticosterone and gene expression in metabolic tissues
- PMID: 38553695
- PMCID: PMC10979634
- DOI: 10.1186/s12860-024-00508-6
Mice lacking DIO3 exhibit sex-specific alterations in circadian patterns of corticosterone and gene expression in metabolic tissues
Abstract
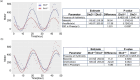
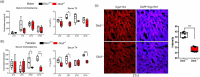
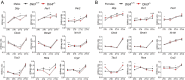
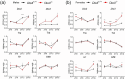
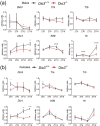
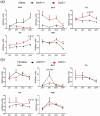
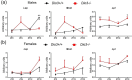
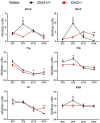
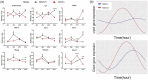
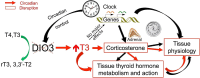
Disruption of circadian rhythms is associated with neurological, endocrine and metabolic pathologies. We have recently shown that mice lacking functional type 3 deiodinase (DIO3), the enzyme that clears thyroid hormones, exhibit a phase shift in locomotor activity, suggesting altered circadian rhythm. To better understand the physiological and molecular basis of this phenotype, we used Dio3+/+ and Dio3-/- mice of both sexes at different zeitgeber times (ZTs) and analyzed corticosterone and thyroxine (T4) levels, hypothalamic, hepatic, and adipose tissue expression of clock genes, as well as genes involved in the thyroid hormone action or physiology of liver and adipose tissues. Wild type mice exhibited sexually dimorphic circadian patterns of genes controlling thyroid hormone action, including Dio3. Dio3-/- mice exhibited altered hypothalamic expression of several clock genes at ZT12, but did not disrupt the overall circadian profile. Expression of clock genes in peripheral tissues was not disrupted by Dio3 deficiency. However, Dio3 loss in liver and adipose tissues disrupted circadian profiles of genes that determine tissue thyroid hormone action and physiology. We also observed circadian-specific changes in serum T4 and corticosterone as a result of DIO3 deficiency. The circadian alterations manifested sexual dimorphism. Most notable, the time curve of serum corticosterone was flattened in Dio3-/- females. We conclude that Dio3 exhibits circadian variations, influencing the circadian rhythmicity of thyroid hormone action and physiology in liver and adipose tissues in a sex-specific manner. Circadian disruptions in tissue physiology may then contribute to the metabolic phenotypes of DIO3-deficient mice.
Keywords: Adipose tissue; Circadian rhythm; Clock genes; Corticosterone; Hypothalamus; Liver; Thyroid hormone action; Type 2 deiodinase; Type 3 deiodinase.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Type 3 Deiodinase Role on Central Thyroid Hormone Action Affects the Leptin-Melanocortin System and Circadian Activity.Endocrinology. 2017 Feb 1;158(2):419-430. doi: 10.1210/en.2016-1680. Endocrinology. 2017. PMID: 27911598 Free PMC article.
-
Adult onset of type 3 deiodinase deficiency in mice alters brain gene expression and increases locomotor activity.Psychoneuroendocrinology. 2019 Dec;110:104439. doi: 10.1016/j.psyneuen.2019.104439. Epub 2019 Sep 18. Psychoneuroendocrinology. 2019. PMID: 31561084 Free PMC article.
-
Transgenerational epigenetic self-memory of Dio3 dosage is associated with Meg3 methylation and altered growth trajectories and neonatal hormones.Epigenetics. 2024 Dec;19(1):2376948. doi: 10.1080/15592294.2024.2376948. Epub 2024 Jul 11. Epigenetics. 2024. PMID: 38991122 Free PMC article.
-
Genomic imprinting of the type 3 thyroid hormone deiodinase gene: regulation and developmental implications.Biochim Biophys Acta. 2013 Jul;1830(7):3946-55. doi: 10.1016/j.bbagen.2012.03.015. Epub 2012 Apr 4. Biochim Biophys Acta. 2013. PMID: 22498139 Free PMC article. Review.
-
The Type 3 Deiodinase: Epigenetic Control of Brain Thyroid Hormone Action and Neurological Function.Int J Mol Sci. 2018 Jun 19;19(6):1804. doi: 10.3390/ijms19061804. Int J Mol Sci. 2018. PMID: 29921775 Free PMC article. Review.
Cited by
-
Transcriptomic landscape of hyperthyroidism in mice overexpressing thyroid-stimulating hormone.iScience. 2024 Dec 10;28(1):111565. doi: 10.1016/j.isci.2024.111565. eCollection 2025 Jan 17. iScience. 2024. PMID: 39811643 Free PMC article.
-
Thyroid Hormone Clearance in the Paraventricular Nucleus of Male Mice Regulates Lean Mass and Physical Activity.Neuroendocrinology. 2024;114(11):1066-1076. doi: 10.1159/000541525. Epub 2024 Sep 18. Neuroendocrinology. 2024. PMID: 39293416
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

