MANNosylation of Mesoporous Silica Nanoparticles Modifies TLR4 Localization and NF-κB Translocation in T24 Bladder Cancer Cells
- PMID: 38554019
- PMCID: PMC11468387
- DOI: 10.1002/adhm.202304150
MANNosylation of Mesoporous Silica Nanoparticles Modifies TLR4 Localization and NF-κB Translocation in T24 Bladder Cancer Cells
Abstract
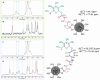
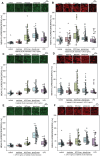
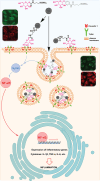
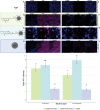
D-mannose is widely used as non-antibiotic treatment for bacterial urinary tract infections. This application is based on a well-studied mechanism of binding to the type 1 bacterial pili and, therefore, blocking bacteria adhesion to the uroepithelial cells. To implement D-mannose into carrier systems, the mechanism of action of the sugar in the bladder environment is also relevant and requires investigation. Herein, two different MANNosylation strategies using mesoporous silica nanoparticles (MSNs) are described. The impact of different chemical linkers on bacterial adhesion and bladder cell response is studied via confocal microscopy imaging of the MSN interactions with the respective organisms. Cytotoxicity is assessed and the expression of Toll-like receptor 4 (TLR4) and caveolin-1 (CAV-1), in the presence or absence of simulated infection with bacterial lipopolysaccharide (LPS), is evaluated using the human urinary bladder cancer cell line T24. Further, localisation of the transcription factor NF-κB due to the MANNosylated materials is examined over time. The results show that MANNosylation modifies bacterial adhesion to the nanomaterials and significantly affects TLR4, caveolin-1, and NF-κB in bladder cells. These elements are essential components of the inflammatory cascade/pathogens response during urinary tract infections. These findings demonstrate that MANNosylation is a versatile tool to design hybrid nanocarriers for targeted biomedical applications.
Keywords: Caveolin 1; MSNs; TLR4; UTIs; bladder cells; immunomodulation; mannose.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Mesoporous Silica Nanoparticles Trigger Liver and Kidney Injury and Fibrosis Via Altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 Signaling in Rats.Biomolecules. 2019 Sep 25;9(10):528. doi: 10.3390/biom9100528. Biomolecules. 2019. PMID: 31557909 Free PMC article.
-
Lactobacillus rhamnosus GR-1 enhances NF-kappaB activation in Escherichia coli-stimulated urinary bladder cells through TLR4.BMC Microbiol. 2012 Jan 22;12:15. doi: 10.1186/1471-2180-12-15. BMC Microbiol. 2012. PMID: 22264349 Free PMC article.
-
Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury in rats by inhibition of caveolin-1 downstream signaling.Biomed Pharmacother. 2019 Oct;118:109314. doi: 10.1016/j.biopha.2019.109314. Epub 2019 Aug 10. Biomed Pharmacother. 2019. PMID: 31545263
-
Mesoporous Silica Nanoparticles as a Potential Nanoplatform: Therapeutic Applications and Considerations.Int J Mol Sci. 2023 Mar 28;24(7):6349. doi: 10.3390/ijms24076349. Int J Mol Sci. 2023. PMID: 37047329 Free PMC article. Review.
-
Mesoporous silica nanoparticles in drug delivery and biomedical applications.Nanomedicine. 2015 Feb;11(2):313-27. doi: 10.1016/j.nano.2014.09.014. Epub 2014 Nov 13. Nanomedicine. 2015. PMID: 25461284 Review.
Cited by
-
Nucleic acid-based drugs for patients with solid tumours.Nat Rev Clin Oncol. 2024 Jun;21(6):407-427. doi: 10.1038/s41571-024-00883-1. Epub 2024 Apr 8. Nat Rev Clin Oncol. 2024. PMID: 38589512 Review.
References
-
- Fan E., Dashti M., Fuentes J., Reitzer L., Christie A. L., Zimmern P. E., Neurourol. Urodyn. 2022, 42, 49. - PubMed
-
- Kranjcec B., Papes D., Altarac S., World J. Urol. 2013, 32, 79. - PubMed
-
- Barea B. M., Veeratterapillay R., Harding C., Curr. Opin. Urol. 2020, 30, 845. - PubMed
-
- Foo L. Y., Lu Y., Howell A. B., Vorsa N., J. Nat. Prod. 2000, 63, 1225. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

