Abnormal Colorectal Cancer Test Follow-Up: A Quality Improvement Initiative at a Federally Qualified Health Center
- PMID: 38554066
- PMCID: PMC10981848
- DOI: 10.1177/21501319241242571
Abnormal Colorectal Cancer Test Follow-Up: A Quality Improvement Initiative at a Federally Qualified Health Center
Abstract
Introduction/objectives: Colonoscopy completion rates after an abnormal fecal immunochemical test (FIT) are suboptimal, resulting in missed opportunities for early detection and prevention of colorectal cancer. Patient navigation and structured follow-up may improve colonoscopy completion, but implementation of these strategies is not widespread.
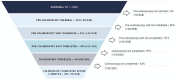
Methods: We conducted a quality improvement study using a Plan-Do-Study-Act (PDSA) Model to increase colonoscopy completion after abnormal FIT in a large federally qualified health center serving a diverse and low-income population. Intervention components included patient navigation, and a checklist to promote completion of key steps required for abnormal FIT follow-up. Primary outcome was proportion of patients achieving colonoscopy completion within 6 months of abnormal FIT, assessed at baseline for 156 patients pre-intervention, and compared to 208 patients during the intervention period from April 2017 to December 2019. Drop offs at each step in the follow-up process were assessed.
Results: Colonoscopy completion improved from 21% among 156 patients with abnormal FIT pre-intervention, to 38% among 208 patients with abnormal FIT during the intervention (P < .001; absolute increase: 17%, 95% CI: 6.9%-25.2%). Among the 130 non-completers during the intervention period, lack of completion was attributable to absence of colonoscopy referral for 7.7%; inability to schedule a pre-colonoscopy specialist visit for 71.5%; failure to complete a pre-colonoscopy visit for 2.3%; the absence of colonoscopy scheduling for 9.2%; failure to show for a scheduled colonoscopy for 9.2%.
Conclusions: Patient navigation and structured follow-up appear to improve colonoscopy completion after abnormal FIT. Additional strategies are needed to achieve optimal rates of completion.
Keywords: abnormal fecal immunochemical test follow-up; care coordination; colorectal cancer screening; federally qualified health center; patient navigation.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Colonoscopy Following an Abnormal Fecal Test Result from an Annual Colorectal Cancer Screening Program in a Federally Qualified Health Center.J Prim Care Community Health. 2022 Jan-Dec;13:21501319221138423. doi: 10.1177/21501319221138423. J Prim Care Community Health. 2022. PMID: 36448466 Free PMC article.
-
Monthly Variations in Colorectal Cancer Screening Tests Among Federally Qualified Health Center Patients in Missouri: Quality Improvement Project.JMIR Cancer. 2025 Mar 19;11:e64809. doi: 10.2196/64809. JMIR Cancer. 2025. PMID: 40106833 Free PMC article.
-
Diagnostic colonoscopy completion after abnormal fecal immunochemical testing and quality of tests used at 8 Federally Qualified Health Centers in Southern California: Opportunities for improving screening outcomes.Cancer. 2019 Dec 1;125(23):4203-4209. doi: 10.1002/cncr.32440. Epub 2019 Sep 3. Cancer. 2019. PMID: 31479529 Free PMC article.
-
Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system.Cancer. 2016 Feb 1;122(3):456-63. doi: 10.1002/cncr.29770. Epub 2015 Nov 4. Cancer. 2016. PMID: 26535565 Free PMC article. Clinical Trial.
-
Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial.JAMA. 2017 Sep 5;318(9):806-815. doi: 10.1001/jama.2017.11389. JAMA. 2017. PMID: 28873161 Free PMC article. Clinical Trial.
References
-
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

