Single-cell adhesive profiling in an optofluidic device elucidates CD8+ T lymphocyte phenotypes in inflamed vasculature-like microenvironments
- PMID: 38554703
- PMCID: PMC11046032
- DOI: 10.1016/j.crmeth.2024.100743
Single-cell adhesive profiling in an optofluidic device elucidates CD8+ T lymphocyte phenotypes in inflamed vasculature-like microenvironments
Abstract
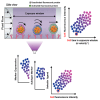
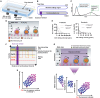
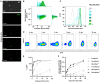
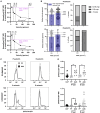
Tissue infiltration by circulating leukocytes occurs via adhesive interactions with the local vasculature, but how the adhesive quality of circulating cells guides the homing of specific phenotypes to different vascular microenvironments remains undefined. We developed an optofluidic system enabling fluorescent labeling of photoactivatable cells based on their adhesive rolling velocity in an inflamed vasculature-mimicking microfluidic device under physiological fluid flow. In so doing, single-cell level multidimensional profiling of cellular characteristics could be characterized and related to the associated adhesive phenotype. When applied to CD8+ T cells, ligand/receptor expression profiles and subtypes associated with adhesion were revealed, providing insight into inflamed tissue infiltration capabilities of specific CD8+ T lymphocyte subsets and how local vascular microenvironmental features may regulate the quality of cellular infiltration. This methodology facilitates rapid screening of cell populations for enhanced homing capabilities under defined biochemical and biophysical microenvironments, relevant to leukocyte homing modulation in multiple pathologies.
Keywords: CP: biotechnology; CP: immunology; cell adhesion; homing; inflamed vasculature; lymphocyte; microfluidic system; photoactivation; selectins; single-cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- He R., Hou S., Liu C., Zhang A., Bai Q., Han M., Yang Y., Wei G., Shen T., Yang X., et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature. 2016;537:412–428. - PubMed
-
- Tietz W., Allemand Y., Borges E., von Laer D., Hallmann R., Vestweber D., Hamann A. CD4+ T cells migrate into inflamed skin only if they express ligands for E-and P-selectin. J. Immunol. 1998;161:963–970. - PubMed
-
- Hirata T., Furie B.C., Furie B. P-E-and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J. Immunol. 2002;169:4307–4313. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

