Effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: an individual participant data meta-analysis
- PMID: 38554713
- PMCID: PMC7615958
- DOI: 10.1016/S2213-8587(24)00040-8
Effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: an individual participant data meta-analysis
Abstract
Background: Previous meta-analyses of summary data from randomised controlled trials have shown that statin therapy increases the risk of diabetes, but less is known about the size or timing of this effect, or who is at greatest risk. We aimed to address these gaps in knowledge through analysis of individual participant data from large, long-term, randomised, double-blind trials of statin therapy.
Methods: We conducted a meta-analysis of individual participant data from randomised controlled trials of statin therapy that participated in the CTT Collaboration. All double-blind randomised controlled trials of statin therapy of at least 2 years' scheduled duration and with at least 1000 participants were eligible for inclusion in this meta-analysis. All recorded diabetes-related adverse events, treatments, and measures of glycaemia were sought from eligible trials. Meta-analyses assessed the effects of allocation to statin therapy on new-onset diabetes (defined by diabetes-related adverse events, use of new glucose-lowering medications, glucose concentrations, or HbA1c values) and on worsening glycaemia in people with diabetes (defined by complications of glucose control, increased use of glucose-lowering medication, or HbA1c increase of ≥0·5%). Standard inverse-variance-weighted meta-analyses of the effects on these outcomes were conducted according to a prespecified protocol.
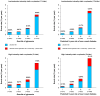
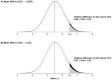
Findings: Of the trials participating in the CTT Collaboration, 19 trials compared statin versus placebo (123 940 participants, 25 701 [21%] with diabetes; median follow-up of 4·3 years), and four trials compared more versus less intensive statin therapy (30 724 participants, 5340 [17%] with diabetes, median follow-up of 4·9 years). Compared with placebo, allocation to low-intensity or moderate-intensity statin therapy resulted in a 10% proportional increase in new-onset diabetes (2420 of 39 179 participants assigned to receive a statin [1·3% per year] vs 2214 of 39 266 participants assigned to receive placebo [1·2% per year]; rate ratio [RR] 1·10, 95% CI 1·04-1·16), and allocation to high-intensity statin therapy resulted in a 36% proportional increase (1221 of 9935 participants assigned to receive a statin [4·8% per year] vs 905 of 9859 participants assigned to receive placebo [3·5% per year]; 1·36, 1·25-1·48). For each trial, the rate of new-onset diabetes among participants allocated to receive placebo depended mostly on the proportion of participants who had at least one follow-up HbA1c measurement; this proportion was much higher in the high-intensity than the low-intensity or moderate-intensity trials. Consequently, the main determinant of the magnitude of the absolute excesses in the two types of trial was the extent of HbA1c measurement rather than the proportional increase in risk associated with statin therapy. In participants without baseline diabetes, mean glucose increased by 0·04 mmol/L with both low-intensity or moderate-intensity (95% CI 0·03-0·05) and high-intensity statins (0·02-0·06), and mean HbA1c increased by 0·06% (0·00-0·12) with low-intensity or moderate-intensity statins and 0·08% (0·07-0·09) with high-intensity statins. Among those with a baseline measure of glycaemia, approximately 62% of new-onset diabetes cases were among participants who were already in the top quarter of the baseline distribution. The relative effects of statin therapy on new-onset diabetes were similar among different types of participants and over time. Among participants with baseline diabetes, the RRs for worsening glycaemia were 1·10 (1·06-1·14) for low-intensity or moderate-intensity statin therapy and 1·24 (1·06-1·44) for high-intensity statin therapy compared with placebo.
Interpretation: Statins cause a moderate dose-dependent increase in new diagnoses of diabetes that is consistent with a small upwards shift in glycaemia, with the majority of new diagnoses of diabetes occurring in people with baseline glycaemic markers that are close to the diagnostic threshold for diabetes. Importantly, however, any theoretical adverse effects of statins on cardiovascular risk that might arise from these small increases in glycaemia (or, indeed, from any other mechanism) are already accounted for in the overall reduction in cardiovascular risk that is seen with statin therapy in these trials. These findings should further inform clinical guidelines regarding clinical management of people taking statin therapy.
Funding: British Heart Foundation, UK Medical Research Council, and Australian National Health and Medical Research Council.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests CR, DP, LB, JE, KD, HH, CH, LH, KW, AJR, RCl, BM, JA, CB, and RCo are affiliated with the Nuffield Department of Population Health, which has an explicit policy of not accepting any personal honoraria payments directly or indirectly from the pharmaceutical and food industries. It only seeks reimbursement to the University of Oxford for the costs of travel and accommodation to participate in scientific meetings (https://www.ndph.ox.ac.uk/about/independence-of-research). CR reports receiving funding to the University of Oxford from the UK National Institute for Health and Care Research Health Technology Assessment (NIHR HTA) Programme (17/140/02) and holding unpaid roles on the Clinical Data Interchange Standards Consortium (CDISC) as a board member and WHO as a scientific advisor. DP reports receiving funding to his research institution (but no personal funding) from Novartis for the ORION 4 trial of inclisiran, Novo Nordisk for the ASCEND PLUS trial of semaglutide, and Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial and being a committee member for National Institute for Health and Care Excellence guideline NG238 (cardiovascular disease: risk assessment and reduction including lipid modification guideline). LB reports being an unpaid CDISC Expert Advisory Board member. AJR reports funding to his research institution from Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial. ES reports now being an employee of AstraZeneca. CPC reports research grants from Bristol-Myers Squibb to his institution for the PROVE IT-TIMI 22 trial; further grants from Amgen, Better Therapeutics, Boehringer-Ingelheim, Daiichi Sankyo, Merck, Novo Nordisk, and Pfizer; receiving consulting fees from Amryt/Chiesi, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, CSL Behring, Eli Lilly, Janssen, Lexicon, Merck, Milestone, Pfizer, Rhoshan, and Sanofi; and participating on data safety monitoring boards (DSMBs) or advisory boards for Applied Therapeutics, Areteia, Novo Nordisk, and the Veterans Administration. HMC reports grants from IQVIA, Juvenile Diabetes Research Foundation, Chief Scientist Office, Diabetes UK, the Medical Research Council (UK Research and Innovation), and the EU Commission; taking part in speaker's bureau at Novo Nordisk; being on advisory boards for Novo Nordisk and Bayer; and being a shareholder in Roche Pharmaceuticals and Bayer. GAH reports funding from Pfizer for the CARDS study. GKH reports research grants from Klinkerpad fonds and the Dutch Science Organisation (Vidi); part-time employment with Novo Nordisk; and owning stock options in Novo Nordisk. JWJ reports departmental research grants from and being a speaker (with or without lecture fees) for meetings supported by Abbott, Amarin, Amgen, Athera, Biotronik, Boston Scientific, Dalcor, Daiichi Sankyo, Edwards Lifesciences, GE Healthcare, Johnson and Johnson, Lilly, Medtronic, Merck-Schering-Plough, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi Aventis, the Netherlands Heart Foundation, CardioVascular Research the Netherlands, the Netherlands Heart Institute, and the European Community Framework KP7 Programme. WK reports grants and provision of reagents to his institution from Singulex, Dr Beckmann Pharma, Abbott, and Roche Diagnostics; receiving consulting fees from AstraZeneca, Novartis, Amgen, Pfizer, The Medicines Company, DalCor Pharmaceuticals, Kowa, Corvidia Therapeutics, OMEICOS, Daichii Sankyo, Novo Nordisk, New Amsterdam Pharma, TenSixteen Bio, Esperion, and Genentech; and lecturing fees from Bristol Myers Squibb, Novartis, Amgen, Berlin-Chemie, Sanofi, and AstraZeneca. IM reports funding to his institution from the National Health and Medical Research Council (NHMRC) of Australia and the Medical Research Future Fund of Australia; receiving royalties from Taylor and Francis publishers for authorship of a textbook; payment to his institution for his expert opinion and educational events from the Therapeutic Goods Administration of Australia; and holding several unpaid DSMB memberships for academic trials. BM reports funding to the University of Oxford from the UK NIHR HTA Programme (17/140/02) and being on the Value in Health Advisory Board, the European Society of Cardiology Clinical Practice Guidelines Committee, and the NIHR Programme Grants for Applied Research subpanel A and NIHR Research for Patient Benefit Methodologists Committee A. CN reports textbook royalties from Elsevier; a one-time honorarium for serving on the Steering Committee of Lipid Center of excellence from Medscape; support for registration from the Endocrine Society; travel expenses for the US Food and Deug Administration Endocrine and Metabolic Drugs Advisory Committee; and being unpaid Vice President to the Medical Women's International Association. PMR reports research grant funding to his institution from AstraZeneca, National Heart, Lung, and Blood Institute, Kowa, Novartis, Pfizer, Esperion, and Novo Nordisk; consulting fees from AstraZeneca, Novartis, Novo Nordisk, Janssen, Flame, Agepha, Ardelyx, Zomagen, Horizon Therapeutics, CSL Behring, Cardio Therapeutics, Civi Biopharm, GlaxoSmithKline, SOCAR, Health Outlook, Montai Health, Eli Lilly, New Amsterdam, Boehringer-Ingelheim, RTI, and Cytokinetics; roles in the Peter Munk Advisory Board (University of Toronto), the Leducq Foundation, Paris FR, and the Baim Institute (Boston, MA); and stock or stock options with Uppton, Bitteroot Bio, and Angiowave. MSS reports research grant support through Brigham and Women's Hospital from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eisai, Intarcia, Ionis, Merck, Novartis, Pfizer, Saghmos Therapeutics, and Verve Therapeutics; consulting fees from Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Boehringer Ingelheim, Dr Reddy's Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, Precision BioSciences, and Silence Therapeutics; and being a member of the Thrombolysis in Myocardial Infarction Study Group, which has received institutional research grant support through Brigham and Women's Hospital from Abiomed, ARCA Biopharma, Janssen Research and Development, MedImmune, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, Softcell Medical, The Medicines Company, and Zora Biosciences. NS reports grants paid to his institution from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics; consulting fees paid via his institution from Abbott Laboratories Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, Novo Nordisk, Pfizer, and Sanofi; personal fees from Merck Sharp and Dohme, Novartis, Roche Diagnostics, and Novo Nordisk; and other payment or honoraria paid via his institution from Abbott Laboratories, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Janssen. GGS reports grants for research support to the University of Colorado from AstraZeneca, Sanofi, Silence Therapeutics, and The Medicines Company; research support from the US Department of Veterans Affairs; and support to attend trial steering Committee meetings from the University of Oxford. LT reports consulting fees from Servier. AT reports participation in the ORION-4 trial DSMB. HW reports grant support paid to his institution for the HEART–FID Study from American Regent, Dal GenE Study from DalCor Pharma UK, AEGIS-II study from CSL Behring, ISCHEMIA and MINT studies from the US National Institutes of Health , CLEAR Outcomes Study from Esperion Therapeutics, SOLIST-WHF and SCORED trials from Sanofi Aventis Australia, and Librexia AF and ACS studies from Janssen; consulting fees for serving on steering committees for the GenE Study from DalCor Pharma UK, the AEGIS-II study from CSL Behring, the SOLIST-WHF and SCORED Trials from Sanofi Aventis Australia, the CLEAR Outcomes Study from Esperion Therapeutics, and the Librexia AF and ACS studies from Janssen; accommodation support paid to self for attendance at the clinical trial forum 2022 and 2023 from SAHMRI; being chair of the DSMB for the EVIDENCE study; and being on the advisory board of CSL Behring. JA reports previous grants to Oxford University for the Medical Research Council/British Heart Foundation Heart Protection Study, SEARCH, and HPS2-THRIVE trials; being an unpaid data monitoring committee member for the Woman2, So START, and ReSTART trials; and being an unpaid chair of the British Heart Foundation Clinical Studies Committee. AK reports salary support from a NHMRC fellowship; research support from a NHMRC programme grant, Abbott, Amgen, Bayer, Mylan, Novartis, and Sanofi; speaker fees from Novartis; participation on Kowa Safety and Data Monitoring Committee; and receiving drugs for research trials from Viatris and Aspen. JS reports research grants to the University of Sydney from the NHMRC Australia; research contracts to the University of Sydney from Bayer, Roche, Bristol Myers Squibb, Astra Zeneca, Amgen, Pfizer, and MSD; consulting or advisory board fees from FivepHusion paid to the University of Sydney; and being an unpaid STAREE DSMB chair. RCo reports being supported by grants to the University of Oxford from the British Heart Foundation, UK Medical Research Council, Merck, Novartis, Cancer Research UK, AstraZeneca, Wellcome Trust, and Regeneron Pharmaceuticals; having a patent for a statin-related myopathy genetic test licensed to University of Oxford from Boston Heart Diagnostics (RCo has waived any personal reward with any share in royalty and other payments waived in favour of the Nuffield Department of Population Health, University of Oxford) and being chair of the PROMINENT trial data monitoring committee, deputy chair of not-for-profit clinical trial company PROTAS, and chief executive of UK Biobank. CB reports receiving funding to his research institution from Boehringer Ingelheim and Eli Lilly for the EMPA-KIDNEY trial; funding from the UK Medical Research Council (Population Health Research Unit Director, Capital award, Therapy Acceleration Laboratory Award); funding for the University of Oxford from the UK NIHR HTA Programme (17/140/02); being coapplicant for a substantive site award received from Health Data Research UK; unpaid DSMB membership for chairing trials run by Merck, the NIHR HTA, and the British Heart Foundation; and being the unpaid chair for work conducted by the European Society of Cardiology and NIHR HTA. All other authors declare no competing interests.
Figures


Comment in
-
How clinically relevant is statin-induced diabetes?Lancet Diabetes Endocrinol. 2024 May;12(5):286-287. doi: 10.1016/S2213-8587(24)00059-7. Epub 2024 Mar 27. Lancet Diabetes Endocrinol. 2024. PMID: 38554714 No abstract available.
-
Risk of new-onset diabetes with high-intensity statin use.Lancet Diabetes Endocrinol. 2024 Sep;12(9):612-613. doi: 10.1016/S2213-8587(24)00217-1. Lancet Diabetes Endocrinol. 2024. PMID: 39174155 No abstract available.
References
-
- Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. - PubMed
-
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

