PPARs in atherosclerosis: The spatial and temporal features from mechanism to druggable targets
- PMID: 38555000
- PMCID: PMC11954843
- DOI: 10.1016/j.jare.2024.03.020
PPARs in atherosclerosis: The spatial and temporal features from mechanism to druggable targets
Abstract
Background: Atherosclerosis is a chronic and complex disease caused by lipid disorder, inflammation, and other factors. It is closely related to cardiovascular diseases, the chief cause of death globally. Peroxisome proliferator-activated receptors (PPARs) are valuable anti-atherosclerosis targets that showcase multiple roles at different pathological stages of atherosclerosis and for cell types at different tissue sites.
Aim of review: Considering the spatial and temporal characteristics of the pathological evolution of atherosclerosis, the roles and pharmacological and clinical studies of PPARs were summarized systematically and updated under different pathological stages and in different vascular cells of atherosclerosis. Moreover, selective PPAR modulators and PPAR-pan agonists can exert their synergistic effects meanwhile reducing the side effects, thereby providing novel insight into future drug development for precise spatial-temporal therapeutic strategy of anti-atherosclerosis targeting PPARs.
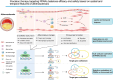
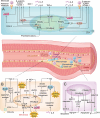
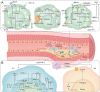
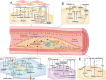
Key scientific: Concepts of Review: Based on the spatial and temporal characteristics of atherosclerosis, we have proposed the importance of stage- and cell type-dependent precision therapy. Initially, PPARs improve endothelial cells' dysfunction by inhibiting inflammation and oxidative stress and then regulate macrophages' lipid metabolism and polarization to improve fatty streak. Finally, PPARs reduce fibrous cap formation by suppressing the proliferation and migration of vascular smooth muscle cells (VSMCs). Therefore, research on the cell type-specific mechanisms of PPARs can provide the foundation for space-time drug treatment. Moreover, pharmacological studies have demonstrated that several drugs or compounds can exert their effects by the activation of PPARs. Selective PPAR modulators (that specifically activate gene subsets of PPARs) can exert tissue and cell-specific effects. Furthermore, the dual- or pan-PPAR agonist could perform a better role in balancing efficacy and side effects. Therefore, research on cells/tissue-specific activation of PPARs and PPAR-pan agonists can provide the basis for precision therapy and drug development of PPARs.
Keywords: Atherosclerosis; Endothelial cells; Macrophages; PPARs; Pharmacology; VSMCs.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Israelian-Konaraki Z., Reaven P.D. Peroxisome proliferator-activated receptor-alpha and atherosclerosis: from basic mechanisms to clinical implications. Cardiol Rev. 2005;13:240–246. - PubMed
-
- Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. - PubMed
-
- Erusalimsky J.D., Skene C. Mechanisms of endothelial senescence. Exp Physiol. 2009;94:299–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

