Combination of acalabrutinib with lenalidomide and rituximab in relapsed/refractory aggressive B-cell non-Hodgkin lymphoma: a single-arm phase II trial
- PMID: 38555311
- PMCID: PMC10981676
- DOI: 10.1038/s41467-024-47198-4
Combination of acalabrutinib with lenalidomide and rituximab in relapsed/refractory aggressive B-cell non-Hodgkin lymphoma: a single-arm phase II trial
Abstract
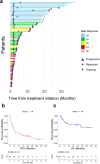
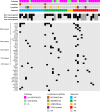
Potential synergism between Bruton's tyrosine kinase (BTK) inhibitor and lenalidomide in treating aggressive B-cell lymphoma has been suggested. Here, the authors report a single-arm phase II clinical trial of combination of acalabrutinib, lenalidomide and rituximab (R2A) in patients with aggressive relapsed/refractory aggressive (R/R) B-cell non-Hodgkin lymphoma (NHL). The primary endpoint of this study is objective response rate (ORR), and the secondary endpoints are complete remission (CR) rate, duration of response (DoR), progression-free survival (PFS) and overall survival (OS). A total of 66 patients are enrolled mostly with diffuse large B-cell lymphoma. The ORR is 54.5% and CR rate is 31.8% meeting the primary end point. The median DoR is 12.9 months, and 1-year PFS and OS rate is 33.1% and 67.5% respectively. Adverse events (AE) are manageable with the most frequent AE being neutropenia (31.8%). Patients with MYD88 mutations, subtypes known for NF-κB activation, and high BTK expression by immunohistochemistry respond well. Overall, these results show a significant efficacy of the R2A regimen in patients with aggressive R/R B-cell NHL, with exploratory biomarkers suggesting potential associations with response. (ClinicalTrials.gov 51 identifier: NCT04094142).
© 2024. The Author(s).
Conflict of interest statement
C.H.S. and Y.K. are founder and stockholder of GenomeOpinion Incorporation. J.L. is employee of GenomeOpinion Incorporation. H.J. and B.C. are employee of PROTEINA corporation. The other authors have no conflict of interest.
Figures

References
-
- Zelenetz, A. D., Gordon, L.I., Abramson, J. S. & Advani, R. H. NCCN Guidelines Version 1.2024; B-Cell Lymphomas. Natl. Compr. Cancer Netw. MS1-118 (2024).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

