Enzymatic conversion of human blood group A kidneys to universal blood group O
- PMID: 38555382
- PMCID: PMC10981661
- DOI: 10.1038/s41467-024-47131-9
Enzymatic conversion of human blood group A kidneys to universal blood group O
Abstract
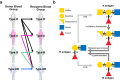
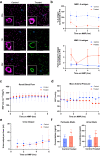
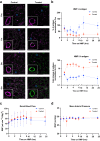
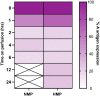
ABO blood group compatibility restrictions present the first barrier to donor-recipient matching in kidney transplantation. Here, we present the use of two enzymes, FpGalNAc deacetylase and FpGalactosaminidase, from the bacterium Flavonifractor plautii to enzymatically convert blood group A antigens from the renal vasculature of human kidneys to 'universal' O-type. Using normothermic machine perfusion (NMP) and hypothermic machine perfusion (HMP) strategies, we demonstrate blood group A antigen loss of approximately 80% in as little as 2 h NMP and HMP. Furthermore, we show that treated kidneys do not bind circulating anti-A antibodies in an ex vivo model of ABO-incompatible transplantation and do not activate the classical complement pathway. This strategy presents a solution to the donor organ shortage crisis with the potential for direct clinical translation to reduce waiting times for patients with end stage renal disease.
© 2024. The Author(s).
Conflict of interest statement
S.G.W., J.N.K., S.Macdonald and P.R. are founders of Avivo Biomedical Inc., which is commercializing the enzymes described. J.N.K., P.R., and S.G.W. are inventors on patent application (PCT no. WO2020034043A1) submitted by the University of British Columbia. The remaining authors declare no competing interests.
Figures

Similar articles
-
A Comparison of Pulsatile Hypothermic and Normothermic Ex Vivo Machine Perfusion in a Porcine Kidney Model.Transplantation. 2021 Aug 1;105(8):1760-1770. doi: 10.1097/TP.0000000000003599. Transplantation. 2021. PMID: 33560723
-
Ex vivo enzymatic treatment converts blood type A donor lungs into universal blood type lungs.Sci Transl Med. 2022 Feb 16;14(632):eabm7190. doi: 10.1126/scitranslmed.abm7190. Epub 2022 Feb 16. Sci Transl Med. 2022. PMID: 35171649
-
Comparison of normothermic and hypothermic perfusion in porcine kidneys donated after cardiac death.J Surg Res. 2017 Aug;216:35-45. doi: 10.1016/j.jss.2017.04.008. Epub 2017 Apr 20. J Surg Res. 2017. PMID: 28807212 Free PMC article.
-
Optimizing porcine donor kidney preservation with normothermic or hypothermic machine perfusion: A systematic review.Artif Organs. 2021 Nov;45(11):1308-1316. doi: 10.1111/aor.14039. Epub 2021 Jul 26. Artif Organs. 2021. PMID: 34309868 Free PMC article.
-
Treatment of transplant kidneys during machine perfusion.Transpl Int. 2021 Feb;34(2):224-232. doi: 10.1111/tri.13751. Epub 2020 Oct 19. Transpl Int. 2021. PMID: 32970886 Review.
Cited by
-
ABO blood groups and galectins: Implications in transfusion medicine and innate immunity.Semin Immunol. 2024 Jul-Sep;74-75:101892. doi: 10.1016/j.smim.2024.101892. Epub 2024 Oct 14. Semin Immunol. 2024. PMID: 39405833 Review.
-
Enzymatic conversion of blood group B kidney prevents hyperacute antibody-mediated injuries in ABO-incompatible transplantation.Nat Commun. 2025 Feb 10;16(1):1506. doi: 10.1038/s41467-025-56563-w. Nat Commun. 2025. PMID: 39929829 Free PMC article.
-
Tolerogenic Therapies in Cardiac Transplantation.Int J Mol Sci. 2025 Apr 23;26(9):3968. doi: 10.3390/ijms26093968. Int J Mol Sci. 2025. PMID: 40362208 Free PMC article. Review.
-
Fluorogenic, Subsingle-Turnover Monitoring of Enzymatic Reactions Involving NAD(P)H Provides a Generalized Platform for Directed Ultrahigh-Throughput Evolution of Biocatalysts in Microdroplets.J Am Chem Soc. 2025 Apr 2;147(13):10903-10915. doi: 10.1021/jacs.4c11804. Epub 2025 Mar 24. J Am Chem Soc. 2025. PMID: 40127491 Free PMC article.
-
The future of nephrology in 2050.Future Healthc J. 2025 Mar 31;12(1):100236. doi: 10.1016/j.fhj.2025.100236. eCollection 2025 Mar. Future Healthc J. 2025. PMID: 40236931 Free PMC article.
References
-
- Annual Activity Report. ODT Clinical - NHS Blood and Transplanthttps://www.odt.nhs.uk/statistics-and-reports/annual-activity-report/.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

