An integrated framework for prognosis prediction and drug response modeling in colorectal liver metastasis drug discovery
- PMID: 38555418
- PMCID: PMC10981831
- DOI: 10.1186/s12967-024-05127-5
An integrated framework for prognosis prediction and drug response modeling in colorectal liver metastasis drug discovery
Abstract
Background: Colorectal cancer (CRC) is the third most prevalent cancer globally, and liver metastasis (CRLM) is the primary cause of death. Hence, it is essential to discover novel prognostic biomarkers and therapeutic drugs for CRLM.
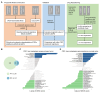
Methods: This study developed two liver metastasis-associated prognostic signatures based on differentially expressed genes (DEGs) in CRLM. Additionally, we employed an interpretable deep learning model utilizing drug sensitivity databases to identify potential therapeutic drugs for high-risk CRLM patients. Subsequently, in vitro and in vivo experiments were performed to verify the efficacy of these compounds.
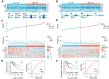
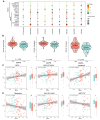
Results: These two prognostic models exhibited superior performance compared to previously reported ones. Obatoclax, a BCL-2 inhibitor, showed significant differential responses between high and low risk groups classified by prognostic models, and demonstrated remarkable effectiveness in both Transwell assay and CT26 colorectal liver metastasis mouse model.
Conclusions: This study highlights the significance of developing specialized prognostication approaches and investigating effective therapeutic drugs for patients with CRLM. The application of a deep learning drug response model provides a new drug discovery strategy for translational medicine in precision oncology.
Keywords: Colorectal liver metastasis; Deep learning; Drug sensitivity; Prognostic biomarker.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Establishment and verification of prognostic model and ceRNA network analysis for colorectal cancer liver metastasis.BMC Med Genomics. 2023 May 9;16(1):99. doi: 10.1186/s12920-023-01523-w. BMC Med Genomics. 2023. PMID: 37161577 Free PMC article.
-
Prediction of Clinical Precision Chemotherapy by Patient-Derived 3D Bioprinting Models of Colorectal Cancer and Its Liver Metastases.Adv Sci (Weinh). 2024 Jan;11(2):e2304460. doi: 10.1002/advs.202304460. Epub 2023 Nov 16. Adv Sci (Weinh). 2024. PMID: 37973557 Free PMC article.
-
Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability.J Hepatobiliary Pancreat Sci. 2013 Feb;20(2):223-33. doi: 10.1007/s00534-012-0531-9. J Hepatobiliary Pancreat Sci. 2013. PMID: 23010994
-
Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases.World J Gastroenterol. 2022 Jan 7;28(1):108-122. doi: 10.3748/wjg.v28.i1.108. World J Gastroenterol. 2022. PMID: 35125822 Free PMC article. Review.
-
Colorectal liver metastasis: molecular mechanism and interventional therapy.Signal Transduct Target Ther. 2022 Mar 4;7(1):70. doi: 10.1038/s41392-022-00922-2. Signal Transduct Target Ther. 2022. PMID: 35246503 Free PMC article. Review.
Cited by
-
Clinical Validation of a Machine Learning-Based Biomarker Signature to Predict Response to Cytotoxic Chemotherapy Alone or Combined with Targeted Therapy in Metastatic Colorectal Cancer Patients: A Study Protocol and Review.Life (Basel). 2025 Feb 19;15(2):320. doi: 10.3390/life15020320. Life (Basel). 2025. PMID: 40003728 Free PMC article.
-
SCGB3A1-Epi and KLK10-Epi Crosstalk With Fibroblasts Promotes Liver Metastasis of Breast Cancer and Pancreatic Ductal Adenocarcinoma.Cancer Med. 2025 May;14(9):e70904. doi: 10.1002/cam4.70904. Cancer Med. 2025. PMID: 40357856 Free PMC article.
References
-
- Yamasaki M, Takemasa I, Komori T, Watanabe S, Sekimoto M, Doki Y, Matsubara K, Monden M. The gene expression profile represents the molecular nature of liver metastasis in colorectal cancer. Int J Oncol. 2007;30(1):129–38. - PubMed
Publication types
MeSH terms
Grants and funding
- 81901687/National Natural Science Foundation of China
- 82002575/National Natural Science Foundation of China
- U20A20369/National Natural Science Foundation of China
- JCYJ20190807154819245/Shenzhen Science and Technology Innovation Program
- KQTD20190929173853397/Shenzhen Science and Technology Innovation Program
LinkOut - more resources
Full Text Sources
Medical
Research Materials

