Progesterone-mediated remodeling of the maternal-fetal interface by a PGRMC1-dependent mechanism
- PMID: 38555747
- PMCID: PMC11151737
- DOI: 10.1016/j.jri.2024.104244
Progesterone-mediated remodeling of the maternal-fetal interface by a PGRMC1-dependent mechanism
Abstract
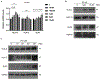
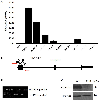
Implantation and maintenance of pregnancy involve intricate immunological processes that enable the developing fetus to coexist with the maternal immune system. Progesterone, a critical hormone during pregnancy, is known to promote immune tolerance and prevent preterm labor. However, the mechanism by which progesterone mediates these effects remains unclear. In this study, we investigated the role of the non-classical progesterone receptor membrane component 1 (PGRMC1) in progesterone signaling at the maternal-fetal interface. Using JEG3 cells, a trophoblast model cell line, we observed that progesterone stimulation increased the expression of human leukocyte antigen-C (HLA-C) and HLA-G, key molecules involved in immune tolerance. We also found that progesterone upregulated the expression of the transcription factor ELF3, which is known to regulate trophoblast-specific HLA-C expression. Interestingly, JEG3 cells lacked expression of classical progesterone receptors (PRs) but exhibited high expression of PGRMC1, a finding we confirmed in primary trophoblasts by mining sc-RNA seq data from human placenta. To investigate the role of PGRMC1 in progesterone signaling, we used CRISPR/Cas9 technology to knockout PGRMC1 in JEG3 cells. PGRMC1-deficient cells showed a diminished response to progesterone stimulation. Furthermore, we found that the progesterone antagonist RU486 inhibited ELF3 expression in a PGRMC1-dependent manner, suggesting that RU486 acts as a progesterone antagonist by competing for receptor binding. Additionally, we found that RU486 inhibited cell invasion, an important process for successful pregnancy, and this inhibitory effect was dependent on PGRMC1. Our findings highlight the crucial role of PGRMC1 in mediating the immunoregulatory effects of progesterone at the maternal-fetal interface.
Keywords: ELF3; HLA-C; HLA-G; Immune tolerance; Maternal-fetal interface; Mifepristone; PGRMC1; Pregnancy; Progesterone; RU486; Trophoblasts.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors report no conflicts of interest.
Figures
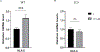

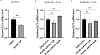
Similar articles
-
A distant trophoblast-specific enhancer controls HLA-G expression at the maternal-fetal interface.Proc Natl Acad Sci U S A. 2016 May 10;113(19):5364-9. doi: 10.1073/pnas.1602886113. Epub 2016 Apr 13. Proc Natl Acad Sci U S A. 2016. PMID: 27078102 Free PMC article.
-
Immunological relationship between the mother and the fetus.Int Rev Immunol. 2002 Nov-Dec;21(6):471-95. doi: 10.1080/08830180215017. Int Rev Immunol. 2002. PMID: 12650238 Review.
-
Roles of HLA-G in the Maternal-Fetal Immune Microenvironment.Front Immunol. 2020 Oct 22;11:592010. doi: 10.3389/fimmu.2020.592010. eCollection 2020. Front Immunol. 2020. PMID: 33193435 Free PMC article. Review.
-
Progesterone-induced progesterone receptor membrane component 1 rise-to-decline changes are essential for decidualization.Reprod Biol Endocrinol. 2024 Feb 3;22(1):20. doi: 10.1186/s12958-024-01188-9. Reprod Biol Endocrinol. 2024. PMID: 38308254 Free PMC article.
-
Downregulation of PGRMC1 accelerates differentiation and fusion of a human trophoblast cell line.J Endocrinol. 2023 Dec 8;260(2):e230163. doi: 10.1530/JOE-23-0163. Print 2024 Feb 1. J Endocrinol. 2023. PMID: 37965940
Cited by
-
Multi-Layered Mechanisms of Immunological Tolerance at the Maternal-Fetal Interface.Immune Netw. 2024 Jul 15;24(4):e30. doi: 10.4110/in.2024.24.e30. eCollection 2024 Aug. Immune Netw. 2024. PMID: 39246621 Free PMC article. Review.
-
The TEA domain transcription factors TEAD1 and TEAD3 and WNT signaling determine HLA-G expression in human extravillous trophoblasts.Proc Natl Acad Sci U S A. 2025 Mar 25;122(12):e2425339122. doi: 10.1073/pnas.2425339122. Epub 2025 Mar 17. Proc Natl Acad Sci U S A. 2025. PMID: 40096597
References
-
- An BS, et al., 2005. Differential role of progesterone receptor isoforms in the transcriptional regulation of human gonadotropin-releasing hormone i (gnrh i) receptor, gnrh i, and gnrh ii. J Clin Endocrinol Metab. 90, 1106–13 - PubMed
-
- Anon, 2021. Evaluating progestogens for preventing preterm birth international collaborative (epppic): Meta-analysis of individual participant data from randomised controlled trials. Lancet (London, England). 397, 1183–1194 - PubMed
-
- Aplin JD, 1991. Implantation, trophoblast differentiation and haemochorial placentation: Mechanistic evidence in vivo and in vitro. Journal of cell science. 99 ( Pt 4), 681–92 - PubMed
-
- Boonyaratanakornkit V, et al., 2001. Progesterone receptor contains a proline-rich motif that directly interacts with sh3 domains and activates c-src family tyrosine kinases. Molecular cell. 8, 269–80 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

