Transcriptomic profiling of sciatic nerves and dorsal root ganglia reveals site-specific effects of prediabetic neuropathy
- PMID: 38556110
- PMCID: PMC11166517
- DOI: 10.1016/j.trsl.2024.03.009
Transcriptomic profiling of sciatic nerves and dorsal root ganglia reveals site-specific effects of prediabetic neuropathy
Abstract
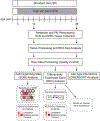
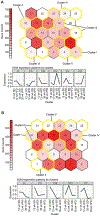
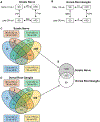
Peripheral neuropathy (PN) is a severe and frequent complication of obesity, prediabetes, and type 2 diabetes characterized by progressive distal-to-proximal peripheral nerve degeneration. However, a comprehensive understanding of the mechanisms underlying PN, and whether these mechanisms change during PN progression, is currently lacking. Here, gene expression data were obtained from distal (sciatic nerve; SCN) and proximal (dorsal root ganglia; DRG) injury sites of a high-fat diet (HFD)-induced mouse model of obesity/prediabetes at early and late disease stages. Self-organizing map and differentially expressed gene analyses followed by pathway enrichment analysis identified genes and pathways altered across disease stage and injury site. Pathways related to immune response, inflammation, and glucose and lipid metabolism were consistently dysregulated with HFD-induced PN, irrespective of injury site. However, regulation of oxidative stress was unique to the SCN while dysregulated Hippo and Notch signaling were only observed in the DRG. The role of the immune system and inflammation in disease progression was supported by an increase in the percentage of immune cells in the SCN with PN progression. Finally, when comparing these data to transcriptomic signatures from human patients with PN, we observed conserved pathways related to metabolic dysregulation across species, highlighting the translational relevance of our mouse data. Our findings demonstrate that PN is associated with distinct site-specific molecular re-programming in the peripheral nervous system, identifying novel, clinically relevant therapeutic targets.
Keywords: Differentially expressed genes; Dorsal root ganglia; High-fat diet; Peripheral neuropathy; Prediabetes; RNA-seq; Sciatic nerve; Self-organizing map.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Lucy M. Hinder is currently employed by Rhythm Pharmaceuticals, but all contributions made by Lucy M. Hinder occurred while she was employed by the University of Michigan. The other authors declare that they have no competing interests. All authors have read the journal's policy on conflicts of interest.
Figures





References
-
- Schnurr TM, et al. , Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: a case-cohort study. Diabetologia, 2020. 63(7): p. 1324–1332. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

