Ceramide kinase-mediated C1P metabolism attenuates acute liver injury by inhibiting the interaction between KEAP1 and NRF2
- PMID: 38556546
- PMCID: PMC11059394
- DOI: 10.1038/s12276-024-01203-4
Ceramide kinase-mediated C1P metabolism attenuates acute liver injury by inhibiting the interaction between KEAP1 and NRF2
Abstract
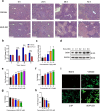
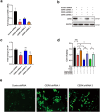
Acute liver injury is the basis of the pathogenesis of diverse liver diseases. However, the mechanism underlying liver injury is complex and not completely understood. In our study, we revealed that CERK, which phosphorylates ceramide to produce ceramide-1-phosphate (C1P), was the sphingolipid pathway-related protein that had the most significantly upregulated expression during acute liver injury. A functional study confirmed that CERK and C1P attenuate hepatic injury both in vitro and in vivo through antioxidant effects. Mechanistic studies have shown that CERK and C1P positively regulate the protein expression of NRF2, which is a crucial protein that helps maintain redox homeostasis. Furthermore, our results indicated that C1P disrupted the interaction between NRF2 and KEAP1 by competitively binding to KEAP1, which allowed for the nuclear translocation of NRF2. In addition, pull-down assays and molecular docking analyses revealed that C1P binds to the DGR domain of KEAP1, which allows it to maintain its interaction with NRF2. Importantly, these findings were verified in human primary hepatocytes and a mouse model of hepatic ischemia‒reperfusion injury. Taken together, our findings demonstrated that CERK-mediated C1P metabolism attenuates acute liver injury via the binding of C1P to the DGR domain of KEAP1 and subsequently the release and nuclear translocation of NRF2, which activates the transcription of cytoprotective and antioxidant genes. Our study suggested that the upregulation of CERK and C1P expression may serve as a potential antioxidant strategy to alleviate acute liver injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

