Selective targeting of dipeptidyl-peptidase 4 (DPP-4) positive senescent chondrocyte ameliorates osteoarthritis progression
- PMID: 38556837
- PMCID: PMC11258469
- DOI: 10.1111/acel.14161
Selective targeting of dipeptidyl-peptidase 4 (DPP-4) positive senescent chondrocyte ameliorates osteoarthritis progression
Abstract
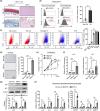
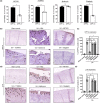
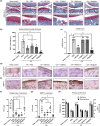
Senescent cells increase in many tissues with age and induce age-related pathologies, including osteoarthritis (OA). Senescent chondrocytes (SnCs) are found in OA cartilage, and the clearance of those chondrocytes prevents OA progression. However, targeting SnCs is challenging due to the absence of a senescent chondrocyte-specific marker. Therefore, we used flow cytometry to screen and select senescent chondrocyte surface markers and cross-validated with published transcriptomic data. Chondrocytes expressing dipeptidyl peptidase-4 (DPP-4), the selected senescent chondrocyte-specific marker, had multiple senescence phenotypes, such as increased senescence-associated-galactosidase, p16, p21, and senescence-associated secretory phenotype expression, and showed OA chondrocyte phenotypes. To examine the effects of DPP-4 inhibition on DPP-4+ SnCs, sitagliptin, a DPP-4 inhibitor, was treated in vitro. As a result, DPP-4 inhibition selectively eliminates DPP-4+ SnCs without affecting DPP-4- chondrocytes. To assess in vivo therapeutic efficacy of targeting DPP-4+ SnCs, three known senolytics (ABT263, 17DMAG, and metformin) and sitagliptin were comparatively verified in a DMM-induced rat OA model. Sitagliptin treatment specifically and effectively eliminated DPP-4+ SnCs, compared to the other three senolytics. Furthermore, Intra-articular sitagliptin injection to the rat OA model increased collagen type II and proteoglycan expression and physical functions and decreased cartilage destruction, subchondral bone plate thickness and MMP13 expression, leading to the amelioration of OA phenotypes. Collectively, OARSI score was lowest in the sitagliptin treatment group. Taken together, we verified DPP-4 as a surface marker for SnCs and suggested that the selective targeting of DPP-4+ chondrocytes could be a promising strategy to prevent OA progression.
Keywords: dipeptidyl peptidase 4; osteoarthritis; senescence; senolytics; sitagliptin.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no relevant financial or non‐financial interests to disclose.
Figures



References
-
- Acosta, J. C. , Banito, A. , Wuestefeld, T. , Georgilis, A. , Janich, P. , Morton, J. P. , Athineos, D. , Kang, T.‐W. , Lasitschka, F. , Andrulis, M. , Pascual, G. , Morris, K. J. , Khan, S. , Jin, H. , Dharmalingam, G. , Snijders, A. P. , Carroll, T. , Capper, D. , Pritchard, C. , … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15(8), 978–990. 10.1038/ncb2784 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

