The six brain-specific TAU isoforms and their role in Alzheimer's disease and related neurodegenerative dementia syndromes
- PMID: 38556838
- PMCID: PMC11095451
- DOI: 10.1002/alz.13784
The six brain-specific TAU isoforms and their role in Alzheimer's disease and related neurodegenerative dementia syndromes
Abstract
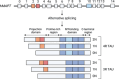
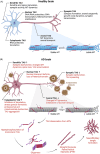
Introduction: Alternative splicing of the human MAPT gene generates six brain-specific TAU isoforms. Imbalances in the TAU isoform ratio can lead to neurodegenerative diseases, underscoring the need for precise control over TAU isoform balance. Tauopathies, characterized by intracellular aggregates of hyperphosphorylated TAU, exhibit extensive neurodegeneration and can be classified by the TAU isoforms present in pathological accumulations.
Methods: A comprehensive review of TAU and related dementia syndromes literature was conducted using PubMed, Google Scholar, and preprint server.
Results: While TAU is recognized as key driver of neurodegeneration in specific tauopathies, the contribution of the isoforms to neuronal function and disease development remains largely elusive.
Discussion: In this review we describe the role of TAU isoforms in health and disease, and stress the importance of comprehending and studying TAU isoforms in both, physiological and pathological context, in order to develop targeted therapeutic interventions for TAU-associated diseases.
Highlights: MAPT splicing is tightly regulated during neuronal maturation and throughout life. TAU isoform expression is development-, cell-type and brain region specific. The contribution of TAU to neurodegeneration might be isoform-specific. Ineffective TAU-based therapies highlight the need for specific targeting strategies.
Keywords: Alzheimer's disease; MAPT; TAU isoforms; alternative splicing; genetic tauopathy; tauopathy.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no competing interest. Author disclosures are available in the supporting information.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

