FOXO1 reshapes neutrophils to aggravate acute brain damage and promote late depression after traumatic brain injury
- PMID: 38556884
- PMCID: PMC10981823
- DOI: 10.1186/s40779-024-00523-w
FOXO1 reshapes neutrophils to aggravate acute brain damage and promote late depression after traumatic brain injury
Abstract
Background: Neutrophils are traditionally viewed as first responders but have a short onset of action in response to traumatic brain injury (TBI). However, the heterogeneity, multifunctionality, and time-dependent modulation of brain damage and outcome mediated by neutrophils after TBI remain poorly understood.
Methods: Using the combined single-cell transcriptomics, metabolomics, and proteomics analysis from TBI patients and the TBI mouse model, we investigate a novel neutrophil phenotype and its associated effects on TBI outcome by neurological deficit scoring and behavioral tests. We also characterized the underlying mechanisms both in vitro and in vivo through molecular simulations, signaling detections, gene expression regulation assessments [including dual-luciferase reporter and chromatin immunoprecipitation (ChIP) assays], primary cultures or co-cultures of neutrophils and oligodendrocytes, intracellular iron, and lipid hydroperoxide concentration measurements, as well as forkhead box protein O1 (FOXO1) conditional knockout mice.
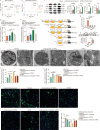
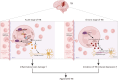
Results: We identified that high expression of the FOXO1 protein was induced in neutrophils after TBI both in TBI patients and the TBI mouse model. Infiltration of these FOXO1high neutrophils in the brain was detected not only in the acute phase but also in the chronic phase post-TBI, aggravating acute brain inflammatory damage and promoting late TBI-induced depression. In the acute stage, FOXO1 upregulated cytoplasmic Versican (VCAN) to interact with the apoptosis regulator B-cell lymphoma-2 (BCL-2)-associated X protein (BAX), suppressing the mitochondrial translocation of BAX, which mediated the antiapoptotic effect companied with enhancing interleukin-6 (IL-6) production of FOXO1high neutrophils. In the chronic stage, the "FOXO1-transferrin receptor (TFRC)" mechanism contributes to FOXO1high neutrophil ferroptosis, disturbing the iron homeostasis of oligodendrocytes and inducing a reduction in myelin basic protein, which contributes to the progression of late depression after TBI.
Conclusions: FOXO1high neutrophils represent a novel neutrophil phenotype that emerges in response to acute and chronic TBI, which provides insight into the heterogeneity, reprogramming activity, and versatility of neutrophils in TBI.
Keywords: Acute stage; Chronic stage; Forkhead box protein O1 (FOXO1); Neutrophil; Traumatic brain injury (TBI).
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Comment in
-
FOXO1-expressing neutrophils: a double-edged sword in traumatic brain injury.Mil Med Res. 2024 Sep 19;11(1):65. doi: 10.1186/s40779-024-00571-2. Mil Med Res. 2024. PMID: 39300550 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

