Electrocatalytic Nitrate and Nitrite Reduction toward Ammonia Using Cu2O Nanocubes: Active Species and Reaction Mechanisms
- PMID: 38557016
- PMCID: PMC11009949
- DOI: 10.1021/jacs.3c13288
Electrocatalytic Nitrate and Nitrite Reduction toward Ammonia Using Cu2O Nanocubes: Active Species and Reaction Mechanisms
Abstract
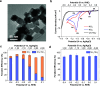
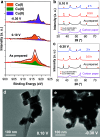
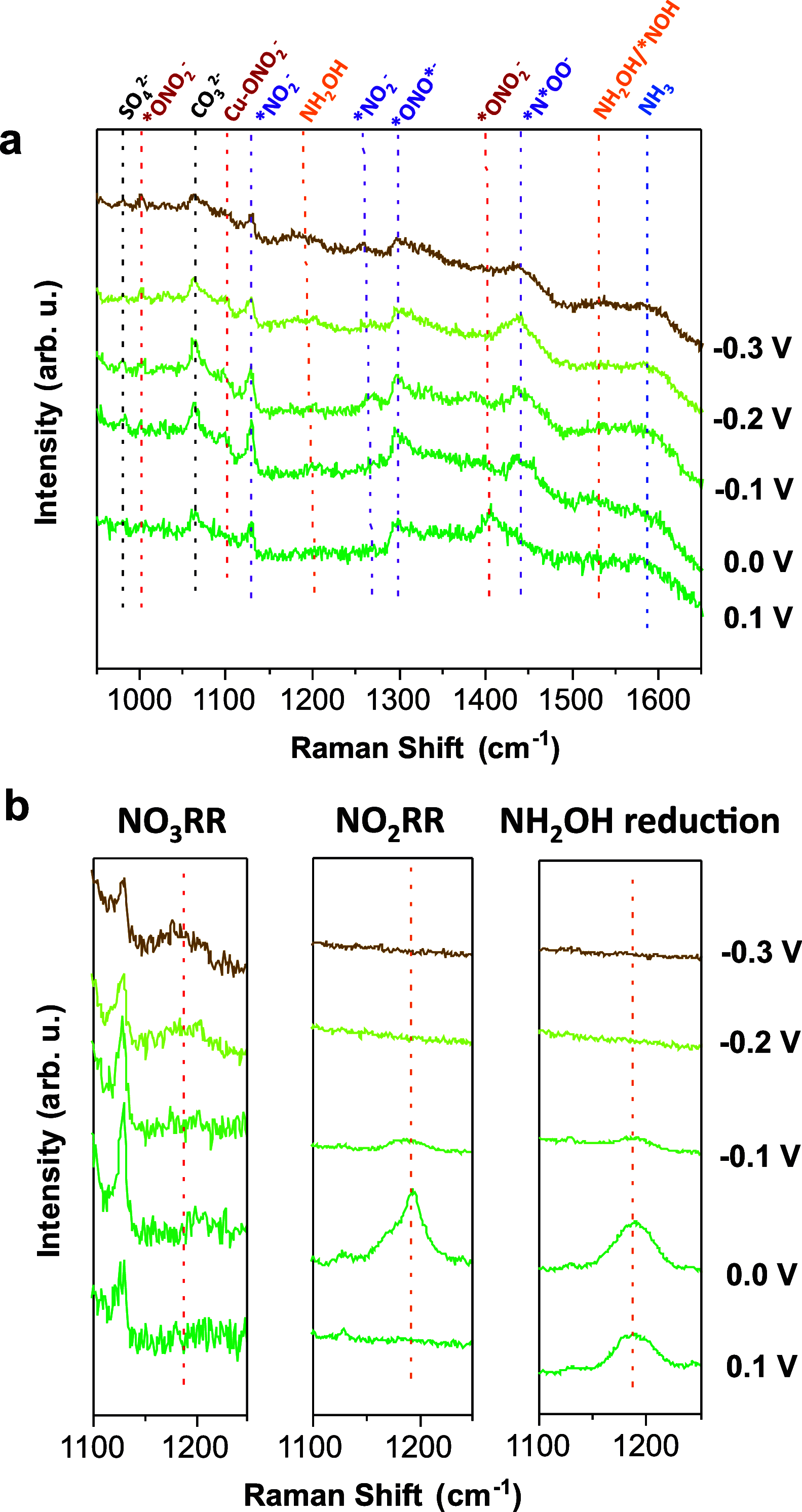
The electrochemical reduction of nitrate (NO3-) and nitrite (NO2-) enables sustainable, carbon-neutral, and decentralized routes to produce ammonia (NH3). Copper-based materials are promising electrocatalysts for NOx- conversion to NH3. However, the underlying reaction mechanisms and the role of different Cu species during the catalytic process are still poorly understood. Herein, by combining quasi in situ X-ray photoelectron spectroscopy (XPS) and operando X-ray absorption spectroscopy (XAS), we unveiled that Cu is mostly in metallic form during the highly selective reduction of NO3-/NO2- to NH3. On the contrary, Cu(I) species are predominant in a potential region where the two-electron reduction of NO3- to NO2- is the major reaction. Electrokinetic analysis and in situ Raman spectroscopy was also used to propose possible steps and intermediates leading to NO2- and NH3, respectively. This work establishes a correlation between the catalytic performance and the dynamic changes of the chemical state of Cu, and provides crucial mechanistic insights into the pathways for NO3-/NO2- electrocatalytic reduction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Wang M.; Khan M. A.; Mohsin I.; Wicks J.; Ip A. H.; Sumon K. Z.; Dinh C.-T.; Sargent E. H.; Gates I. D.; Kibria M. G. Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber-Bosch processes?. Energy Environ. Sci. 2021, 14 (5), 2535–2548. 10.1039/D0EE03808C. - DOI
-
- Wang J.; Feng T.; Chen J.; Ramalingam V.; Li Z.; Kabtamu D. M.; He J.-H.; Fang X. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes. Nano Energy 2021, 86, 106088.10.1016/j.nanoen.2021.106088. - DOI
-
- Wu S.; Salmon N.; Li M. M.-J.; Bañares-Alcántara R.; Tsang S. C. E. Energy Decarbonization via Green H2 or NH3?. ACS Energy Lett. 2022, 7 (3), 1021–1033. 10.1021/acsenergylett.1c02816. - DOI
LinkOut - more resources
Full Text Sources

