Ibrexafungerp is efficacious in a neutropenic murine model of pulmonary mucormycosis as monotherapy and combined with liposomal amphotericin B
- PMID: 38557112
- PMCID: PMC11064560
- DOI: 10.1128/aac.01545-23
Ibrexafungerp is efficacious in a neutropenic murine model of pulmonary mucormycosis as monotherapy and combined with liposomal amphotericin B
Abstract
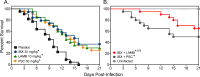
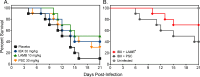
Ibrexafungerp (formerly SCY-078) is the first member of the triterpenoid class that prevents the synthesis of the fungal cell wall polymer β-(1,3)-D-glucan by inhibiting the enzyme glucan synthase. We evaluated the in vivo efficacy of ibrexafungerp against pulmonary mucormycosis using an established murine model. Neutropenic mice were intratracheally infected with either Rhizopus delemar or Mucor circinelloides. Treatment with placebo (diluent control), ibrexafungerp (30 mg/kg, PO BID), liposomal amphotericin B (LAMB 10 mg/kg IV QD), posaconazole (PSC 30 mg/kg PO QD), or a combination of ibrexafungerp plus LAMB or ibrexafungerp plus PSC began 16 h post-infection and continued for 7 days for ibrexafungerp or PSC and through day 4 for LAMB. Ibrexafungerp was as effective as LAMB or PSC in prolonging median survival (range: 15 days to >21 days) and enhancing overall survival (30%-65%) vs placebo (9 days and 0%; P < 0.001) in mice infected with R. delemar. Furthermore, median survival and overall percent survival resulting from the combination of ibrexafungerp plus LAMB were significantly greater compared to all monotherapies (P ≤ 0.03). Similar survival results were observed in mice infected with M. circinelloides. Monotherapies also reduce the lung and brain fungal burden by ~0.5-1.0log10 conidial equivalents (CE)/g of tissue vs placebo in mice infected with R. delemar (P < 0.05), while a combination of ibrexafungerp plus LAMB lowered the fungal burden by ~0.5-1.5log10 CE/g compared to placebo or any of the monotherapy groups (P < 0.03). These results are promising and warrant continued investigation of ibrexafungerp as a novel treatment option against mucormycosis.
Keywords: Ibrexafungerp; Rhizopus; combination therapy; infection model; liposomal amphotericin B; mouse; mucor; mucormycosis; posaconazole.
Conflict of interest statement
A.S.I. has received research support from and served on advisory boards for Astellas, Basilea, Cidara, Matinas, Navigen, Pfizer, and Sfunga. N.P.W. has received support (financial and material) from bioMerieux, Bruker, F2G, Maxwell Biosciences, Mycovia, and Sfunga and has served on an advisory board for F2G. K.B.-E. is an employee of Scynexis. All other authors declare no conflict of interest.
Figures

Similar articles
-
Efficacy of an oral lipid nanocrystal formulation of amphotericin B (MAT2203) in the neutropenic mouse model of pulmonary mucormycosis.Antimicrob Agents Chemother. 2024 Jun 5;68(6):e0154023. doi: 10.1128/aac.01540-23. Epub 2024 Apr 30. Antimicrob Agents Chemother. 2024. PMID: 38687015 Free PMC article.
-
Monotherapy or combination therapy of isavuconazole and micafungin for treating murine mucormycosis.J Antimicrob Chemother. 2017 Feb;72(2):462-466. doi: 10.1093/jac/dkw433. Epub 2016 Oct 24. J Antimicrob Chemother. 2017. PMID: 27798213 Free PMC article.
-
Combination treatment of liposomal amphotericin B and isavuconazole is synergistic in treating experimental mucormycosis.J Antimicrob Chemother. 2021 Sep 15;76(10):2636-2639. doi: 10.1093/jac/dkab233. J Antimicrob Chemother. 2021. PMID: 34263306 Free PMC article.
-
Successful nonoperative management of gastrointestinal mucormycosis: novel therapy for invasive disease.Surg Infect (Larchmt). 2009 Oct;10(5):447-51. doi: 10.1089/sur.2008.049. Surg Infect (Larchmt). 2009. PMID: 19485785 Review.
-
Use of isavuconazole in mucormycosis: a systematic review.BMC Infect Dis. 2025 Jan 6;25(1):25. doi: 10.1186/s12879-025-10439-y. BMC Infect Dis. 2025. PMID: 39762765 Free PMC article.
Cited by
-
Ibrexafungerp prolongs survival and reduces the eumycetoma grain size in Galleria mellonella infection models of two different causative agents of eumycetoma.J Antimicrob Chemother. 2025 Jun 3;80(6):1733-1741. doi: 10.1093/jac/dkaf133. J Antimicrob Chemother. 2025. PMID: 40304084 Free PMC article.
-
Diagnosis and management of invasive fungal infections due to non-Aspergillus moulds.J Antimicrob Chemother. 2025 Mar 14;80(Supplement_1):i17-i39. doi: 10.1093/jac/dkaf005. J Antimicrob Chemother. 2025. PMID: 40085540 Free PMC article. Review.

