Specific circulating miRNAs are associated with plasma lipids in a healthy American cohort
- PMID: 38557280
- PMCID: PMC11368566
- DOI: 10.1152/physiolgenomics.00087.2023
Specific circulating miRNAs are associated with plasma lipids in a healthy American cohort
Abstract
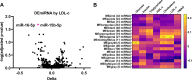
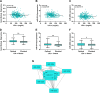
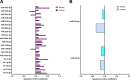
Low-density lipoprotein cholesterol (LDL-c) is both a therapeutic target and a risk factor for cardiovascular disease (CVD). MicroRNA (miRNA) has been shown to regulate cholesterol homeostasis, and miRNA in blood circulation has been linked to hypercholesterolemia. However, few studies to date have associated miRNA with phenotypes like LDL-c in a healthy population. To this end, we analyzed circulating miRNA in relation to LDL-c in a healthy cohort of 353 participants using two separate bioinformatic approaches. The first approach found that miR-15b-5p and miR-16-5p were upregulated in individuals with at-risk levels of LDL-c. The second approach identified two miRNA clusters, one that positively and a second that negatively correlated with LDL-c. Included in the cluster that positively correlated with LDL-c were miR-15b-5p and miR-16-5p, as well as other miRNA from the miR-15/107, miR-30, and let-7 families. Cross-species analyses suggested that several miRNAs that associated with LDL-c are conserved between mice and humans. Finally, we examined the influence of diet on circulating miRNA. Our results robustly linked circulating miRNA with LDL-c, suggesting that miRNA could be used as biomarkers for hypercholesterolemia or targets for developing cholesterol-lowering drugs.NEW & NOTEWORTHY This study explored the association between circulating microRNA (miRNA) and low-density lipoprotein cholesterol (LDL-c) in a healthy population of 353 participants. Two miRNAs, miR-15b-5p and miR-16-5p, were upregulated in individuals with at-risk LDL-c levels. Several miRNA clusters were positively and negatively correlated with LDL-c and are known to target mRNA involved in lipid metabolism. The study also investigated the influence of diet on circulating miRNA, suggesting potential biomarkers for hypercholesterolemia.
Keywords: LDL-c; diet; miRNA; microRNA.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

