IL-6-mediated endothelial injury impairs antiviral humoral immunity after bone marrow transplantation
- PMID: 38557487
- PMCID: PMC10977988
- DOI: 10.1172/JCI174184
IL-6-mediated endothelial injury impairs antiviral humoral immunity after bone marrow transplantation
Abstract
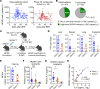
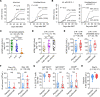
Endothelial function and integrity are compromised after allogeneic bone marrow transplantation (BMT), but how this affects immune responses broadly remains unknown. Using a preclinical model of CMV reactivation after BMT, we found compromised antiviral humoral responses induced by IL-6 signaling. IL-6 signaling in T cells maintained Th1 cells, resulting in sustained IFN-γ secretion, which promoted endothelial cell (EC) injury, loss of the neonatal Fc receptor (FcRn) responsible for IgG recycling, and rapid IgG loss. T cell-specific deletion of IL-6R led to persistence of recipient-derived, CMV-specific IgG and inhibited CMV reactivation. Deletion of IFN-γ in donor T cells also eliminated EC injury and FcRn loss. In a phase III clinical trial, blockade of IL-6R with tocilizumab promoted CMV-specific IgG persistence and significantly attenuated early HCMV reactivation. In sum, IL-6 invoked IFN-γ-dependent EC injury and consequent IgG loss, leading to CMV reactivation. Hence, cytokine inhibition represents a logical strategy to prevent endothelial injury, thereby preserving humoral immunity after immunotherapy.
Keywords: Bone marrow transplantation; Cytokines; Immunoglobulins; Transplantation.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

