Soluble immune checkpoint factors reflect exhaustion of antitumor immunity and response to PD-1 blockade
- PMID: 38557498
- PMCID: PMC10977985
- DOI: 10.1172/JCI168318
Soluble immune checkpoint factors reflect exhaustion of antitumor immunity and response to PD-1 blockade
Abstract
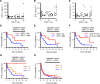
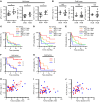
BACKGROUNDPrecise stratification of patients with non-small cell lung cancer (NSCLC) is needed for appropriate application of PD-1/PD-L1 blockade therapy.METHODSWe measured soluble forms of the immune-checkpoint molecules PD-L1, PD-1, and CTLA-4 in plasma of patients with advanced NSCLC before PD-1/PD-L1 blockade. A prospective biomarker-finding trial (cohort A) included 50 previously treated patients who received nivolumab. A retrospective observational study was performed for patients treated with any PD-1/PD-L1 blockade therapy (cohorts B and C), cytotoxic chemotherapy (cohort D), or targeted therapy (cohort E). Plasma samples from all patients were assayed for soluble immune-checkpoint molecules with a highly sensitive chemiluminescence-based assay.RESULTSNonresponsiveness to PD-1/PD-L1 blockade therapy was associated with higher concentrations of these soluble immune factors among patients with immune-reactive (hot) tumors. Such an association was not apparent for patients treated with cytotoxic chemotherapy or targeted therapy. Integrative analysis of tumor size, PD-L1 expression in tumor tissue (tPD-L1), and gene expression in tumor tissue and peripheral CD8+ T cells revealed that high concentrations of the 3 soluble immune factors were associated with hyper or terminal exhaustion of antitumor immunity. The combination of soluble PD-L1 (sPD-L1) and sCTLA-4 efficiently discriminated responsiveness to PD-1/PD-L1 blockade among patients with immune-reactive tumors.CONCLUSIONCombinations of soluble immune factors might be able to identify patients unlikely to respond to PD-1/PD-L1 blockade as a result of terminal exhaustion of antitumor immunity. Our data suggest that such a combination better predicts, along with tPD-L1, for the response of patients with NSCLC.TRIAL REGISTRATIONUMIN000019674.FUNDINGThis study was funded by Ono Pharmaceutical Co. Ltd. and Sysmex Corporation.
Keywords: Immunotherapy; Lung cancer; Oncology; T cells.
Figures




Comment in
- Soluble immune-checkpoint factors: a potential immunotherapy biomarker doi: 10.1172/JCI179352
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

