Axicabtagene ciloleucel vs standard of care in second-line large B-cell lymphoma: outcomes by metabolic tumor volume
- PMID: 38557775
- PMCID: PMC11208295
- DOI: 10.1182/blood.2023021620
Axicabtagene ciloleucel vs standard of care in second-line large B-cell lymphoma: outcomes by metabolic tumor volume
Abstract
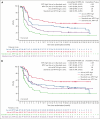
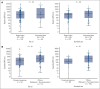
Metabolic tumor volume (MTV) assessed using 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography, a measure of tumor burden, is a promising prognostic indicator in large B-cell lymphoma (LBCL). This exploratory analysis evaluated relationships between baseline MTV (categorized as low [median or less] vs high [greater than median]) and clinical outcomes in the phase 3 ZUMA-7 study (NCT03391466). Patients with LBCL relapsed within 12 months of or refractory to first-line chemoimmunotherapy were randomized 1:1 to axicabtagene ciloleucel (axi-cel; autologous anti-CD19 chimeric antigen receptor T-cell therapy) or standard care (2-3 cycles of chemoimmunotherapy followed by high-dose chemotherapy with autologous stem cell transplantation in patients who had a response). All P values are descriptive. Within high- and low-MTV subgroups, event-free survival (EFS) and progression-free survival (PFS) were superior with axi-cel vs standard care. EFS in patients with high MTV (vs low MTV) was numerically shorter with axi-cel and was significantly shorter with standard care. PFS was shorter in patients with high MTV vs low MTV in both the axi-cel and standard-care arms, and median MTV was lower in patients in ongoing response at data cutoff vs others. Median MTV was higher in patients treated with axi-cel who experienced grade ≥3 neurologic events or cytokine release syndrome (CRS) than in patients with grade 1/2 or no neurologic events or CRS, respectively. Baseline MTV less than or equal to median was associated with better clinical outcomes in patients receiving axi-cel or standard care for second-line LBCL. The trial was registered at www.clinicaltrials.gov as #NCT03391466.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: F.L.L. reports consulting/advisory role for Allogene, Amgen, bluebird bio, Bristol Myers Squibb, Celgene, Calibr, Cellular Biomedicine Group, Cowen, EcoR1 Capital, Emerging Therapy Solutions Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite, Legend Biotech, Novartis, Umoja Biopharma, and Wugen; research funding from Allogene, Kite, and Novartis; and patents, royalties, and other intellectual property from several patents held by the institution in their name (unlicensed) in the field of cellular immunotherapy. O.O.O. reports research funding from Kite; and consulting/advisory role for Janssen, Pfizer, Novartis, Curio Science, ADC Therapeutics, and TG Therapeutics. J.K. reports honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Gilead, Janssen, Karyopharm, Merck, Novartis, Roche, and Seagen; consulting/advisory role for AbbVie, Bristol Myers Squibb, Gilead, Karyopharm, Merck, Roche, and Seagen; and research funding from Roche and Janssen. C. Thieblemont reports honoraria from and consulting/advisory role for AbbVie, Bristol Myers Squibb, Celgene, Incyte, Kite, Novartis, Roche, and Takeda; and travel support from Bristol Myers Squibb, Celgene, Kite, Novartis, Roche, and Takeda. F.M. reports consulting/advisory role for AbbVie, Bristol Myers Squibb, Epizyme, Genmab, Gilead Sciences, Novartis, and Roche; speakers’ bureau participation for Roche; and expert testimony for Roche and Genentech. G.S. reports honoraria from AbbVie, Amgen, Bayer, Epizyme, Regeneron, Roche, MorphoSys, Kite, and Novartis; consultancy/advisory role for Bristol Myers Squibb, Celgene, Incyte, Ipsen, Janssen, Kite, Loxo, Miltenyi Biotec, MorphoSys, Novartis, and Rapt; participation on a data safety monitoring board or advisory board for AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, Miltenyi Biotec, MorphoSys, Takeda, and VelosBio. S.P.R. reports employment with, stock, or other ownership in and patents, royalties, other intellectual property from Precision Molecular and PlenaryAI; honoraria from, speakers’ bureau participation for, and travel support from Lantheus Pharmaceuticals; and consultancy/advisory role for and research funding from Precision Molecular, Lantheus Pharmaceuticals, and PlenaryAI. S.V. reports employment with and research funding from Kite; and stock or other ownership in Gilead Sciences. J.W. reports employment with and research funding from Kite; and stock or other ownership in Gilead. S.F. reports employment and stock or ownership with Kite; and patents, royalties, and other intellectual property from Tusk Therapeutics. C. To reports employment with Kite; and stock or other ownership in Gilead Sciences. P.C. reports employment with Kite; stock or other ownership in Gilead Sciences; and travel support from Kite. M.S. reports employment with, honoraria from, travel support from, and other relationships with Kite; and stock or other ownership in Gilead Sciences. R.K. reports employment with Imaging Endpoints; stock or other ownership in Teladoc, Teleview, Globavir, Verve, and Renibus; consulting or advisory role for Dynamicure, Fore Biotherapeutics, SonALAsense, Dracen, Day One, and FibroGen; and patents, royalties, and other intellectual property with Imaging Endpoints. M.J.K. reports honoraria from and consulting/advisory role for Bristol Myers Squibb, Celgene, Kite, Miltenyi Biotech, Novartis, and Roche; research funding from Kite, Roche, Takeda, and Celgene; and travel support from Kite, Miltenyi Biotech, Novartis, and Roche.
Figures


Comment in
-
Tumor burden in ZUMA-7: less is more.Blood. 2024 Jun 13;143(24):2441-2442. doi: 10.1182/blood.2024024592. Blood. 2024. PMID: 38869918 No abstract available.
References
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540–1545. - PubMed
-
- Nastoupil LJ, Bartlett NL. Navigating the evolving treatment landscape of diffuse large B-cell lymphoma. J Clin Oncol. 2023;41(4):903–913. - PubMed
-
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

