Determination, expression and characterization of an UDP-N-acetylglucosamine:α-1,3-D-mannoside β-1,2-N-acetylglucosaminyltransferase I (GnT-I) from the Pacific oyster, Crassostrea gigas
- PMID: 38557922
- PMCID: PMC11065688
- DOI: 10.1007/s10719-024-10148-9
Determination, expression and characterization of an UDP-N-acetylglucosamine:α-1,3-D-mannoside β-1,2-N-acetylglucosaminyltransferase I (GnT-I) from the Pacific oyster, Crassostrea gigas
Abstract
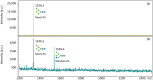
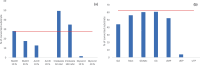
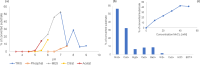
Molluscs are intermediate hosts for several parasites. The recognition processes, required to evade the host's immune response, depend on carbohydrates. Therefore, the investigation of mollusc glycosylation capacities is of high relevance to understand the interaction of parasites with their host. UDP-N-acetylglucosamine:α-1,3-D-mannoside β-1,2-N-acetylglucosaminyltransferase I (GnT-I) is the key enzyme for the biosynthesis of hybrid and complex type N-glycans catalysing the transfer of N-acetylglucosamine from UDP-N-acetylglucosamine to the α-1,3 Man antenna of Man5GlcNAc2. Thereby, the enzyme produces a suitable substrate for further enzymes, such as α-mannosidase II, GlcNAc-transferase II, galactosyltransferases or fucosyltransferases. The sequence of GnT- I from the Pacific oyster, Crassostrea gigas, was obtained by homology search using the corresponding human enzyme as the template. The obtained gene codes for a 445 amino acids long type II transmembrane glycoprotein and shared typical structural elements with enzymes from other species. The enzyme was expressed in insect cells and purified by immunoprecipitation using protein A/G-plus agarose beads linked to monoclonal His-tag antibodies. GnT-I activity was determined towards the substrates Man5-PA, MM-PA and GnM-PA. The enzyme displayed highest activity at pH 7.0 and 30 °C, using Man5-PA as the substrate. Divalent cations were indispensable for the enzyme, with highest activity at 40 mM Mn2+, while the addition of EDTA or Cu2+ abolished the activity completely. The activity was also reduced by the addition of UDP, UTP or galactose. In this study we present the identification, expression and biochemical characterization of the first molluscan UDP-N-acetylglucosamine:α-1,3-D-mannoside β-1,2-N-acetylglucosaminyltransferase I, GnT-I, from the Pacific oyster Crassostrea gigas.
Keywords: Crassostrea gigas; Glycosyltransferase; GnT-I; Mollusca; N-glycosylation; UDP-N-acetylglucosamine:α-1,3-D-mannoside β-1,2-N-acetylglucosaminyltransferase I.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

