Safety of Deutetrabenazine for the Treatment of Tardive Dyskinesia and Chorea Associated with Huntington Disease
- PMID: 38557959
- PMCID: PMC11136929
- DOI: 10.1007/s40120-024-00600-1
Safety of Deutetrabenazine for the Treatment of Tardive Dyskinesia and Chorea Associated with Huntington Disease
Erratum in
-
Correction: Safety of Deutetrabenazine for the Treatment of Tardive Dyskinesia and Chorea Associated with Huntington Disease.Neurol Ther. 2024 Dec;13(6):1747-1749. doi: 10.1007/s40120-024-00660-3. Neurol Ther. 2024. PMID: 39266813 Free PMC article. No abstract available.
Abstract
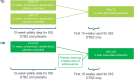
Introduction: Deutetrabenazine is a vesicular monoamine transporter 2 inhibitor used to treat tardive dyskinesia (TD) and chorea associated with Huntington disease (HD). To enhance detection of safety signals across individual trials, integrated safety analyses of deutetrabenazine in TD and HD chorea were conducted.
Methods: For TD, safety data were integrated from two 12-week pivotal studies (ARM-TD and AIM-TD) and through week 15 of the open-label extension (OLE) study (RIM-TD). Data were analyzed by deutetrabenazine treatment group and placebo. For HD, safety data were integrated from the 12-week pivotal study (First-HD) and through week 15 of the OLE study (ARC-HD) for patients previously receiving placebo. Integrated deutetrabenazine data were compared with placebo from the pivotal study.
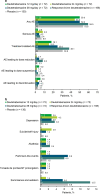
Results: For TD, deutetrabenazine (n = 384) was generally well tolerated compared with placebo (n = 130). Adverse event (AE) incidence was numerically higher in the response-driven deutetrabenazine vs the fixed-dose deutetrabenazine and placebo groups, respectively (any AE, 59.5% vs 44.4-50.0% and 53.8%; treatment-related AE, 38.1% vs 18.1-25.0% and 30.8%). Serious AEs were reported for 2.8-8.3% of patients in the deutetrabenazine groups and 6.9% in the placebo group. Common AEs (≥ 4%) included headache, somnolence, nausea, anxiety, fatigue, dry mouth, and diarrhea. AE incidence was higher during the titration vs maintenance periods. For HD, AE incidence was numerically higher with deutetrabenazine (n = 84) vs placebo (n = 45; any AE, 64.3% vs 60.0%; treatment-related AE, 38.1% vs 26.7%); serious AEs were reported for similar proportions for the deutetrabenazine and placebo groups, 2.4% and 2.2%, respectively. Common AEs (≥ 4%) included irritability, fall, depression, dry mouth, and fatigue.
Conclusions: Data from an integrated analysis of studies in TD and an integrated analysis of studies of chorea in HD showed that deutetrabenazine has a favorable safety profile and is well tolerated across indications.
Trial registration: ClinicalTrials.gov identifiers, NCT02291861, NCT02195700, NCT01795859, NCT02198794, NCT01897896.
Keywords: Chorea; Deutetrabenazine; Huntington disease; Movement disorders; Safety profile; Tardive dyskinesia; Tolerability.
Plain language summary
Unintended movements are often the first sign of Huntington disease. This type of unintended movement is called chorea in Huntington disease. Tardive dyskinesia causes unintended body movements. Deutetrabenazine is a medicine used to treat both types of movements. This report summarizes deutetrabenazine safety across five clinical studies. Safety was assessed via adverse events (side effects). Adverse events were compared between deutetrabenazine and inactive treatment (placebo). Serious adverse events were also compared. Serious adverse events cause substantial impairment or disruption. In tardive dyskinesia and chorea in Huntington disease studies, most patients kept taking deutetrabenazine. Adverse events were not a common reason to stop treatment. For tardive dyskinesia, adverse event rates were similar between deutetrabenazine (≤ 60%) and placebo (54%). Serious adverse event rates were also similar for deutetrabenazine (≤ 8%) and placebo (7%). Adverse events tended to be reported earlier in treatment. Common adverse events were headache, sleepiness, nausea, anxiety, fatigue, dry mouth, and diarrhea. For chorea in Huntington disease, adverse event rates were similar for deutetrabenazine (64%) and placebo (60%). Serious adverse event rates were also similar for deutetrabenazine (2%) and placebo (2%). Irritability, fall, depression, dry mouth, and fatigue were common adverse events. Adverse events were similar between deutetrabenazine and placebo in both conditions. Deutetrabenazine was well tolerated for patients with either tardive dyskinesia or chorea in Huntington disease.
© 2024. The Author(s).
Conflict of interest statement
Samuel Frank is principal investigator of First-HD and ARC-HD (Teva), consultant for Novartis, Sage Therapeutics, and uniQure, and has received research support from Cure Huntington’s Disease Initiative Foundation, Huntington’s Disease Society of America, the Huntington Study Group, Neurocrine, Roche/Genentech, and Triplet Therapeutics. Karen E. Anderson is global principal/co-principal investigator of AIM-TD and ARM-TD (Teva Pharmaceuticals; funds paid directly to her institution, Georgetown University), North American study co-principal investigator of LEGATO-HD (Teva), site principal investigator of Pride-HD, First-HD, and ARC-HD (Teva), ENROLL-HD (Cure Huntington’s Disease Initiative Foundation), Vaccinex, and site investigator for Generation HD studies (Roche Biogen). She is a consultant for NeuroNEXT 105 study (Azevan) and scientific advisor for Azevan, Biogen, Cure Huntington’s Disease Initiative Foundation, Novartis, Prana, and Roche. She has received salary support from The Griffin Foundation and Honoraria from Vindico Medical Education. Hubert H. Fernandez has received honoraria from AbbVie, Biogen, Carling Communications, GE Health Care, inVentiv, International Parkinson and Movement Disorders Society, Kyowa Hakko Kirin, Lundbeck, Medscape (speaker in CME events), Merz Pharmaceuticals, Pfizer (as a consultant), Prime Education, Sunovion, and Voyager Therapeutics. He has been provided grant and research support by AbbVie, Acadia, Biotie/Acorda, Civitas, Kyowa/ProStrakan, Michael J. Fox Foundation, Movement Disorders Society, National Institute of Health/National Institute of Neurological Disorders and Stroke, Parkinson Study Group, Rhythm Pharmaceuticals, Synosia, Teva Pharmaceuticals. He has received royalties from Demos Publishing (serving as a book author/editor) and contractual services with The Cleveland Clinic via a contract with Teva for Dr Fernandez’s role as co-principal investigator in SD-809 tardive dyskinesia global studies. Dr Fernandez also serves as a member of the publication committee for Acorda, but does not receive any personal compensation for this. He has received a stipend from the International Parkinson and Movement Disorders Society for serving as medical editor for the MDS website. Robert A. Hauser has served as a consultant with AbbVie, Academy for Continued Healthcare Learning, Acorda, Adamas Pharmaceuticals, AstraZeneca, Back Bay Life Science, Biotie, ClearView Healthcare Partners, ClinicalMind Medical and Therapeutic Communications, Cowen and Company, Cynapsus, eResearch Technology, Expert Connect, Gerson Lehrman Group, Guidepoint Global, Health Advances, HealthLogiX, Impax Laboratories, Kyowa Kirin Pharmaceutical Development, LCN Consulting, LifeMax, Lilly, Lundbeck, Michael J. Fox Foundation, National Institutes of Health, National Parkinson Foundation, Neurocrine, Neuropore, Outcomes Insights, PeerView Press, Pfizer, Pharma Two B Ltd., Prexton, Projects in Knowledge, Putnam Associates, Quintiles, RMEI Medical Education for Better Outcomes, Sarepta, Sunovion, Teva Pharmaceuticals, US WorldMeds, and Vista Research. Daniel O. Claassen has received research support from Griffin Foundation, Huntington’s Disease Society of America, Michael J. Fox Foundation, National Institute of Neurological Disorders and Stroke, and National Institute on Aging, and grant support from AbbVie, Acadia, Biogen, Bristol Myers Squibb, Cerecor, Jazz Pharmaceuticals, Lilly, Lundbeck, Teva Pharmaceuticals, Vaccinex, and Wave Life Sciences. Dr Claassen is a consultant/scientific advisor for Acadia, Adamas Pharmaceuticals, Alterity, Lundbeck, Neurocrine, Teva Pharmaceuticals, and Wave Life Sciences. David Stamler is a former employee of Teva Pharmaceuticals. He receives salary and benefits from Auspex Pharmaceuticals. Patents pending: US20160346270, US20160287574. Stewart A. Factor has received honoraria from Acorda, Biogen, CereSpir, Neurocrine, Lundbeck, Teva Pharmaceuticals, Avanir, UCB, US WorldMeds, Sunovion, and Adamas Pharmaceuticals. He has received research support from Biohaven, Boston Scientific, Impax, Ipsen, Jazz Pharmaceuticals, Lilly, Medtronic, National Institutes of Health (U10 NS077366), Neurocrine, Parkinson Foundation, Sun Pharmaceuticals Advanced Research Company, Voyager Therapeutics, Auspex, US WorldMeds, Pharm-Olam, Cynapsus/Sunovion, Vaccinex, Solstice, Cure Huntington’s Disease Initiative Foundation, Michael J. Fox Foundation. He has received royalties from Demos, Blackwell Futura, Springer (textbooks), Uptodate. He has other conflicts of interest with Signant Health (Bracket Global LLC) and CNS Ratings. Joohi Jimenez-Shahed has received consulting fees from AbbVie, Blue Rock Therapeutics, Bracket Global, Medtronic, Nuvelution, Photopharmics, St. Jude Medical, Teva Pharmaceuticals. Hadas Barkay is an employee of Teva Pharmaceutical Industries Ltd. Amanda Wilhelm and Jessica K. Alexander are former employees of Teva Branded Pharmaceutical Products R&D, Inc. Nayla Chaijale, Steve Barash, Mark Forrest Gordon, and Maria Chen are employees of Teva Branded Pharmaceutical Products R&D, Inc. Juha-Matti Savola is a former employee of Teva Pharmaceuticals International GmbH, Basel, Switzerland.
Figures



References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

