Comparison of the Efficacy Between the Dual Therapy of Tegoprazan and the Quadruple Therapy of Tegoprazan: A Randomized Controlled Multicenter Study
- PMID: 38557975
- PMCID: PMC11500782
- DOI: 10.14309/ctg.0000000000000699
Comparison of the Efficacy Between the Dual Therapy of Tegoprazan and the Quadruple Therapy of Tegoprazan: A Randomized Controlled Multicenter Study
Abstract
Introduction: Tegoprazan (TPZ), a potassium-competitive acid blocker, exerts a strong acid-suppression effect and a rapid onset of action. However, research on TPZ-amoxicillin (TA) dual treatment is limited. Here, we compared the safety and efficacy of TPZ-amoxicillin dual treatment and TPZ, bismuth potassium citrate, amoxicillin, and clarithromycin (TBAC) quadruple therapy in patients newly diagnosed with H. pylori infection over a 14-day treatment period.
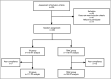
Methods: A total of 236 patients newly diagnosed with H. pylori were enrolled in this multicenter, prospective, open-label, and randomized controlled study. Patients randomly received either TA dual or TBAC quadruple therapy. The incidence of adverse reactions and treatment compliance were recorded and then analyzed.
Results: The intention-to-treat analysis revealed that H. pylori eradication rates were 83.9% (95% confidence interval 78.2%-91.3%) and 81.4% (95% confidence interval 74.2%-88.5%) for the TA and TBAC groups, respectively, with no statistically significant difference between them ( P = 0.606). The per-protocol analysis revealed that the H. pylori eradication rates were 88.3% and 84.8% for the TA and TBAC groups, respectively ( P = 0.447). The incidence of adverse reactions was significantly lower in the TA group than in the TBAC group (4.2% vs 15.3%, P = 0.004). Moreover, the TA group demonstrated substantially higher treatment compliance than the TBAC group (94.1% vs 89.0%, P = 0.020).
Discussion: The TA dual therapy successfully eradicated H. pylori with a high eradication rate and a low incidence of adverse reactions. Therefore, this treatment is recommended as an alternative course for patients newly diagnosed with H. pylori infection.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Figures
References
-
- Massarrat S. Unacceptability of Kyoto global consensus report on Helicobacter pylori gastritis. Arch Iran Med 2016;19(7):527–8. - PubMed
-
- Hooi JK, Lai WY, Ng WK, et al. . Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017;153(2):420–9. - PubMed
-
- Crowe SE. Helicobacter pylori infection. N Engl J Med 2019;380(12):1158–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical