New strain Brevibacillus laterosporus TSA31-5 produces both brevicidine and brevibacillin, exhibiting distinct antibacterial modes of action against Gram-negative and Gram-positive bacteria
- PMID: 38558002
- PMCID: PMC10984550
- DOI: 10.1371/journal.pone.0294474
New strain Brevibacillus laterosporus TSA31-5 produces both brevicidine and brevibacillin, exhibiting distinct antibacterial modes of action against Gram-negative and Gram-positive bacteria
Abstract
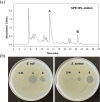
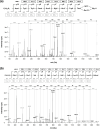
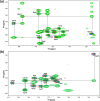
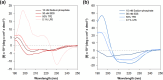
The growing prevalence of antibiotic resistance has made it imperative to search for new antimicrobial compounds derived from natural products. In the present study, Brevibacillus laterosporus TSA31-5, isolated from red clay soil, was chosen as the subject for conducting additional antibacterial investigations. The fractions exhibiting the highest antibacterial activity (30% acetonitrile eluent from solid phase extraction) were purified through RP-HPLC. Notably, two compounds (A and B) displayed the most potent antibacterial activity against both Escherichia coli and Staphylococcus aureus. ESI-MS/MS spectroscopy and NMR analysis confirmed that compound A corresponds to brevicidine and compound B to brevibacillin. Particularly, brevicidine displayed notable antibacterial activity against Gram-negative bacteria, with a minimum inhibitory concentration (MIC) range of 1-8 μg/mL. On the other hand, brevibacillin exhibited robust antimicrobial effectiveness against both Gram-positive bacterial strains (MIC range of 2-4 μg/mL) and Gram-negative bacteria (MIC range of 4-64 μg/mL). Scanning electron microscopy analysis and fluorescence assays uncovered distinctive morphological alterations in bacterial cell membranes induced by brevicidine and brevibacillin. These observations imply distinct mechanisms of antibacterial activity exhibited by the peptides. Brevicidine exhibited no hemolysis or cytotoxicity up to 512 μg/mL, comparable to the negative control. This suggests its promising therapeutic potential in treating infectious diseases. Conversely, brevibacillin demonstrated elevated cytotoxicity in in vitro assays. Nonetheless, owing to its noteworthy antimicrobial activity against pathogenic bacteria, brevibacillin could still be explored as a promising antimicrobial agent.
Copyright: © 2024 Kim et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Mijović G. Antibiotic susceptibility of Salmonella spp.: a comparison of two surveys with a 5 years interval. Journal of IMAB–Annual Proceeding Scientific Papers. 2012;18(1):216–9.
-
- http://www.cdc.gov/drugresistance/ (accessed 23.05.2023) [Internet]. 2019.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

