Early Incorporation to Palliative Care (EPC) in Patients With Advanced Non-Small Cell Lung Cancer: The PACO Randomized Clinical Trial
- PMID: 38558247
- PMCID: PMC11449095
- DOI: 10.1093/oncolo/oyae050
Early Incorporation to Palliative Care (EPC) in Patients With Advanced Non-Small Cell Lung Cancer: The PACO Randomized Clinical Trial
Abstract
Background: Patients with non-small cell lung cancer (NSCLC) experience a considerable disease burden, evident in symptomatic and psychological spheres. Advanced cancer represents a complex scenario for patients and the healthcare team. Early palliative care (EPC) has been proven as a clinically meaningful strategy in this context by several randomized trials but not in a resource-limited setting. This study aimed to evaluate the effect of EPC compared with standard oncological care (SOC) in patients with metastatic NSCLC in Mexico.
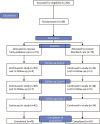
Materials and methods: A prospective, randomized clinical trial was conducted at Instituto Nacional de Cancerologia in Mexico. All patients had histologically confirmed metastatic NSCLC without previous treatment. Patients were randomly assigned (1:1) to receive SOC or SOC + EPC. The EPC group was introduced to the palliative care team at baseline after randomization, which was integrated by psychologists, bachelor's in nutrition, specialized nurses, and physicians. Patients randomized to this arm had programmed visits to meet with the team at baseline and through the 2nd, 4th-, and 6th cycles thereafter. The primary endpoint was overall survival (OS); secondary outcomes included quality of life (QoL), anxiety and depression, and symptom intensity. They were assessed using the instruments EORTC QLQ-C30 questionnaire, Edmonton Symptom Assessment Scale (ESAS), and the Hospital Anxiety and Depression Scale (HADS) (clinicaltrials.gov [NCT01631565]). Questionnaires were completed at baseline, at 2nd, 4th, and 6th cycles of treatment.
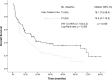
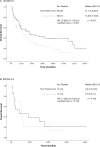
Results: Between March 2012 and June 2015, 201 patients were assessed for eligibility and 146 were enrolled and allocated to receive EPC (73) or SOC (73). Median OS for patients in the EPC vs SOC arm was 18.1 months (95% CI, 7.9-28.4) and 10.5 months (95% CI, 4.7-16.2) (P = .029). Having a poor performance status (HR 1.7 [1.2-2.5]; P = .004) and allocation to the control group (HR 1.5 [1.03-2.3]; P = .034) were independently associated with a worse OS. Those patients with a global QoL > 70 at baseline had a better OS if they were In the EPC arm (38.7 months (95% CI, 9.9-67.6) vs SOC 21.4 months (95% CI, 12.4-30.3)). Mean QoL had a numerical improvement in patients allocated to EPC after 6 cycles of follow-up, nonetheless this difference was not statistically significant (55.1 ± 23.7 vs 56.9 ± 25.3; P = .753). There were no significant differences in anxiety and depression at all study points.
Conclusions: EPC is associated with a significant improvement in OS, although, we observed that the greatest benefit of providing EPC was observed in those with a global QoL > 70 at baseline. This study did not identify significant changes in terms of QoL or symptom burden between the study groups after follow-up. Evidence robustly suggests that EPC should be considered part of the multidisciplinary treatment of metastatic NSCLC patients since diagnosis. According to our study, EPC can be implemented in low- or middle-income countries (LMIC).
Keywords: anxiety; depression; palliative care; quality of life; survival.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Andrés F. Cardona reported grants or contracts from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Foundation Medicine, Roche Diagnostics, Termo Fisher, Broad Institute, Amgen, Flatiron Health, Teva Pharma, Rochem Biocare, Bayer, INQBox, and The Foundation for Clinical and Applied Cancer Research (FICMAC); honoraria from EISAI, Merck Serono, Jannsen Pharmaceutical, Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly, Guardant Health, Illumina, and Foundation for Clinical and Applied Cancer Research (FICMAC); expert testimony for Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Foundation Medicine, Guardant Health, Illumina, and Foundation for Clinical and Applied Cancer Research (FICMAC); travel expenses from Merck Serono, Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly, and Foundation for Clinical and Applied Cancer Research (FICMAC); participation on a Data Safety Monitoring Board or Advisory Board for Roche and Merck Sharp & Dohme; and receipt of equipment, materials, drugs medical writing, gifts or other services from Roche, Roche Diagnostics, and Rochem Biocare. Dr. Oscar Arrieta has received honoraria as an advisor, participated in the speakers’ bureau, and gave expert opinions to Pfizer, AstraZeneca, Boehringer-Ingelheim, Roche, Lilly, and Bristol-Myers Squibb. The other authors indicated no financial relationships.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

