TACE inhibition: a promising therapeutic intervention against AATF-mediated steatohepatitis to hepatocarcinogenesis
- PMID: 38558505
- PMCID: PMC11306524
- DOI: 10.1002/1878-0261.13646
TACE inhibition: a promising therapeutic intervention against AATF-mediated steatohepatitis to hepatocarcinogenesis
Abstract
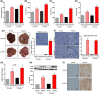
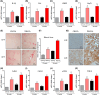
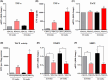
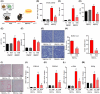
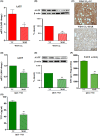
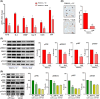
Metabolic dysfunction-associated steatohepatitis-driven hepatocellular carcinoma (MASH-HCC) is a global clinical challenge for which there is a limited understanding of disease pathogenesis and a subsequent lack of therapeutic interventions. We previously identified that tumor necrosis factor-alpha (TNF-α) upregulated apoptosis antagonizing transcription factor (AATF) in MASH. Here, we investigated the effect of TNF-α converting enzyme (TACE) inhibition as a promising targeted therapy against AATF-mediated steatohepatitis to hepatocarcinogenesis. A preclinical murine model that recapitulates human MASH-HCC was used in the study. C57Bl/6 mice were fed with chow diet normal water (CD) or western diet sugar water (WD) along with a low dose of carbon tetrachloride (CCl4; 0.2 μL·g-1, weekly) for 24 weeks. TACE activity, TNF-α levels, and AATF expression were measured. The mice were treated with the TACE inhibitor Marimastat for 12 weeks, followed by analyses of liver injury, fibrosis, inflammation, and oncogenic signaling. In vitro experiments using stable clones of AATF control and AATF knockdown were also conducted. We found that AATF expression was upregulated in WD/CCl4 mice, which developed severe MASH at 12 weeks and advanced fibrosis with HCC at 24 weeks. WD/CCl4 mice showed increased TACE activity with reduced hepatic expression of sirtuin 1 (Sirt1) and tissue inhibitor of metalloproteinase 3 (Timp3). The involvement of the SIRT1/TIMP3/TACE axis was confirmed by the release of TNF-α, which upregulated AATF, a key molecular driver of MASH-HCC. Interestingly, TACE inhibition by Marimastat reduced liver injury, dyslipidemia, AATF expression, and oncogenic signaling, effectively preventing hepatocarcinogenesis. Furthermore, Marimastat inhibited the activation of JNK, ERK1/2, and AKT, which are key regulators of tumorigenesis in WD/CCl4 mice and in AATF control cells, but had no effect on AATF knockdown cells. This study shows that TACE inhibition prevents AATF-mediated inflammation, fibrosis, and oncogenesis in MASH-HCC, offering a potential target for therapeutic intervention.
Keywords: Apoptosis antagonizing transcription factor; Marimastat; hepatocellular carcinoma; metabolic dysfunction associated steatohepatitis; tumor necrosis factor (TNF)‐alpha converting enzyme.
© 2024 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi‐society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–1986. - PubMed
-
- Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16(6):377–386. - PubMed
-
- Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwälder M, el‐Serag HB, et al. Nonalcoholic steatohepatitis‐related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023;20(8):487–503. - PubMed
MeSH terms
Substances
Grants and funding
- SPG/2021/002524/Science and Engineering Research Board (SERB)
- BT/RLF/Reentry/58/2017/Department of Biotechnology, Ministry of Science and Technology, India
- BT/INF/22/SP43045/2021/Department of Biotechnology, Ministry of Science and Technology, India
- 5/3/8/55/2020-ITR/Indian Council of Medical Research (ICMR)
- CR-FST-LS-1/2018/178/Department of Science and Technology (DST)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

