Risk of candidiasis associated with interleukin-17 inhibitors: Implications and management
- PMID: 38558839
- PMCID: PMC10977001
- DOI: 10.1080/21501203.2023.2265664
Risk of candidiasis associated with interleukin-17 inhibitors: Implications and management
Abstract
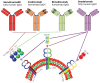
The application of interleukin-17 (IL-17) inhibitors, including secukinumab, ixekizumab, brodalumab, and bimekizumab, are associated with elevated risk of candidiasis. These medications interfere with the IL-17 pathway, which is essential for maintaining mucosal barriers and coordinating the immune response against Candida species. The observational data and clinical trials demonstrate the increased incidence of candidiasis in individuals treated with IL-17 inhibitors. Brodalumab and bimekizumab pose a greater risk than secukinumab in eliciting candidiasis, whereas the data regarding ixekizumab are equivocal. Higher doses and prolonged treatment duration of IL-17 inhibitors increase the risk of candidiasis by compromising the immune response against Candida species. Prior to prescribing IL-17 inhibitors, healthcare professionals should comprehensively evaluate patients' medical histories and assess their risk factors. Patients should be educated on the signs and symptoms of candidiasis to facilitate early detection and intervention. Future research should focus on identifying the risk factors associated with candidiasis in patients receiving IL-17 inhibitors. Prospective studies and long-term surveillance are required to explore the impact of specific inhibitors on the incidence and severity of candidiasis and to evaluate the effectiveness of combination therapies, such as concurrent use of IL-17 inhibitors and prophylactic antifungal agents.
Keywords: Candidiasis; IL-17 inhibitors; bimekizumab; brodalumab; ixekizumab; secukinumab.
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Interleukin inhibitors and the associated risk of candidiasis.Front Immunol. 2024 Mar 28;15:1372693. doi: 10.3389/fimmu.2024.1372693. eCollection 2024. Front Immunol. 2024. PMID: 38605952 Free PMC article. Review.
-
Secukinumab, ixekizumab, bimekizumab and brodalumab for psoriasis and psoriatic arthritis.Drugs Today (Barc). 2023 Mar;59(3):135-167. doi: 10.1358/dot.2023.59.3.3419557. Drugs Today (Barc). 2023. PMID: 36847624 Review.
-
Drug Survival of Interleukin (IL)‑17 and IL‑23 Inhibitors for the Treatment of Psoriasis: A Retrospective Multi‑country, Multicentric Cohort Study.Am J Clin Dermatol. 2022 Nov;23(6):891-904. doi: 10.1007/s40257-022-00722-y. Epub 2022 Aug 17. Am J Clin Dermatol. 2022. PMID: 35976568
-
Bimekizumab treatment in patients with moderate to severe plaque psoriasis: a drug safety evaluation.Expert Opin Drug Saf. 2023 Jan-Jun;22(5):355-362. doi: 10.1080/14740338.2023.2218086. Epub 2023 Jun 19. Expert Opin Drug Saf. 2023. PMID: 37222656 Review.
-
Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources.Lancet Reg Health Eur. 2021 Nov 22;13:100266. doi: 10.1016/j.lanepe.2021.100266. eCollection 2022 Feb. Lancet Reg Health Eur. 2021. PMID: 34950923 Free PMC article.
Cited by
-
The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer.Cancers (Basel). 2024 Aug 28;16(17):2997. doi: 10.3390/cancers16172997. Cancers (Basel). 2024. PMID: 39272855 Free PMC article. Review.
-
Deciphering the role of IL17RA in psoriasis and chronic mucocutaneous candidiasis: shared pathways and distinct manifestations.Front Immunol. 2025 Jan 20;15:1516408. doi: 10.3389/fimmu.2024.1516408. eCollection 2024. Front Immunol. 2025. PMID: 39911581 Free PMC article.
-
Recurrent Vulvovaginal Candidosis and Its Underlying Mechanisms: A Systematic Review.J Fungi (Basel). 2025 May 5;11(5):357. doi: 10.3390/jof11050357. J Fungi (Basel). 2025. PMID: 40422691 Free PMC article. Review.
-
Interleukin inhibitors and the associated risk of candidiasis.Front Immunol. 2024 Mar 28;15:1372693. doi: 10.3389/fimmu.2024.1372693. eCollection 2024. Front Immunol. 2024. PMID: 38605952 Free PMC article. Review.
-
Friend or foe? Sebaceous cyst inflammation during ixekizumab therapy in psoriatic arthritis: case based review.Rheumatol Int. 2025 Aug 12;45(9):193. doi: 10.1007/s00296-025-05949-6. Rheumatol Int. 2025. PMID: 40794191 Free PMC article. Review.
References
-
- Armstrong AW, Blauvelt A, Mrowietz U, Strober B, Gisondi P, Merola JF, Langley RG, Ståhle M, Lebwohl M, Netea MG, et al. 2022. A practical guide to the management of oral candidiasis in patients with plaque psoriasis receiving treatments that target interleukin-17. Dermatol Ther (Heidelb). 12(3):787–800. doi:10.1007/s13555-022-00687-0. - DOI - PMC - PubMed
-
- Azadeh H, Alizadeh-Navaei R, Rezaiemanesh A, Rajabinejad M. 2022. Immune-related adverse events (irAes) in ankylosing spondylitis (AS) patients treated with interleukin (IL)-17 inhibitors: A systematic review and meta-analysis. Inflammopharmacol. 30(2):435–451. doi: 10.1007/s10787-022-00933-z. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
