Water Promotes Melting of a Metal-Organic Framework
- PMID: 38558915
- PMCID: PMC10976635
- DOI: 10.1021/acs.chemmater.3c02873
Water Promotes Melting of a Metal-Organic Framework
Abstract
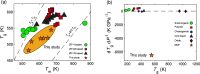
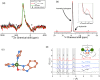
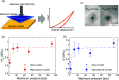
Water is one of the most reactive and abundant molecules on Earth, and it is thus crucial to understand its reactivity with various material families. One of the big unknown questions is how water in liquid and vapor forms impact the fast-emerging class of metal-organic frameworks (MOFs). Here, we discover that high-pressure water vapor drastically modifies the structure and hence the dynamic, thermodynamic, and mechanical properties of MOF glasses. In detail, we find that an archetypical MOF (ZIF-62) is extremely sensitive to heat treatments performed at 460 °C and water vapor pressures up to ∼110 bar. Both the melting and glass transition temperatures decrease remarkably (by >100 °C), and simultaneously, hardness and Young's modulus increase by up to 100% under very mild treatment conditions (<20 bar of hydrothermal pressure). Structural analyses suggest water to partially coordinate to Zn in the form of a hydroxide ion by replacing a bridging imidazolate-based linker. The work provides insight into the role of hot-compressed water in influencing the structure and properties of MOF glasses and opens a new route for systematically changing the thermodynamics and kinetics of MOF liquids and thus altering the thermal and mechanical properties of the resulting MOF glasses.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Banerjee R.; Phan A.; Wang B.; Knobler C.; Furukawa H.; O’Keeffe M.; Yaghi O. M.; Banerjee R.; Phan A.; Wang B.; Carolyn Knobler H. F.; O’Keeffe M.; Y O. M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319 (5865), 939–943. 10.1126/science.1152516. - DOI - PubMed
-
- Bennett T. D.; Yue Y.; Li P.; Qiao A.; Tao H.; Greaves N. G.; Richards T.; Lampronti G. I.; Redfern S. A. T.; Blanc F.; Farha O. K.; Hupp J. T.; Cheetham A. K.; Keen D. A. Melt-Quenched Glasses of Metal-Organic Frameworks. J. Am. Chem. Soc. 2016, 138 (10), 3484–3492. 10.1021/jacs.5b13220. - DOI - PubMed
LinkOut - more resources
Full Text Sources
