Thymic NK-Cells and Their Potential in Cancer Immunotherapy
- PMID: 38558927
- PMCID: PMC10979679
- DOI: 10.2147/ITT.S441639
Thymic NK-Cells and Their Potential in Cancer Immunotherapy
Abstract
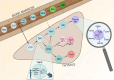
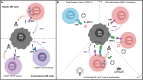
Natural killer (NK)-cells are innate immune cells with potent anti-tumor capacity, capable of recognizing target cells without prior exposure. For this reason, NK-cells are recognized as a useful source of cell therapy. Although most NK-cells are derived from the bone marrow (BM), a separate developmental pathway in the thymus also exists, producing so-called thymic NK-cells. Unlike conventional NK-cells, thymic NK (tNK)-cells have a combined capacity for cytokine production and a natural ability to kill tumor cells in the presence of NK-cell receptor stimulatory ligands. Furthermore, tNK-cells are reported to express CD3 subunits intracellularly, without the presence of a rearranged T-cell receptor (TCR). This unique feature may enable harnessing of these cells with a TCR to combine NK- and T-cell effector properties in one cell type. The development, phenotype, and function of tNK-cells, and potential as a cell therapy is, however, poorly explored. In this review, we provide an overview of current literature on both murine and human tNK-cells in comparison to conventional BM-derived NK-cells, and discuss the potential applications of this cellular subset in the context of cancer immunotherapy.
Keywords: T-cell receptor; cancer immunotherapy; gene engineering; tumor immunogenicity.
© 2024 Forbes et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Evidence for extrathymic development of TNK cells. NK1+ CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice.J Immunol. 1992 Jul 15;149(2):403-12. J Immunol. 1992. PMID: 1385604
-
The dual-functional capability of cytokine-induced killer cells and application in tumor immunology.Hum Immunol. 2015 May;76(5):385-91. doi: 10.1016/j.humimm.2014.09.021. Epub 2014 Oct 8. Hum Immunol. 2015. PMID: 25305457 Review.
-
Murine thymic NK cells are distinct from ILC1s and have unique transcription factor requirements.Eur J Immunol. 2017 May;47(5):800-805. doi: 10.1002/eji.201646871. Epub 2017 Apr 13. Eur J Immunol. 2017. PMID: 28276053 Free PMC article.
-
Bone marrow versus thymic pathways of natural killer cell development.Immunol Rev. 2006 Dec;214:35-46. doi: 10.1111/j.1600-065X.2006.00461.x. Immunol Rev. 2006. PMID: 17100874 Review.
-
Thymic Development of a Unique Bone Marrow-Resident Innate-like T Cell Subset with a Potent Innate Immune Function.J Immunol. 2019 Jul 1;203(1):167-177. doi: 10.4049/jimmunol.1900111. Epub 2019 May 13. J Immunol. 2019. PMID: 31085589
Cited by
-
Ex vivo-generated lymphoid progenitors encompass both T cell and innate lymphoid cell fates.Front Immunol. 2025 Jul 23;16:1617707. doi: 10.3389/fimmu.2025.1617707. eCollection 2025. Front Immunol. 2025. PMID: 40771806 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

