This is a preprint.
State-dependent central synaptic regulation by GLP-1 is essential for energy homeostasis
- PMID: 38559032
- PMCID: PMC10980146
- DOI: 10.21203/rs.3.rs-3929981/v1
State-dependent central synaptic regulation by GLP-1 is essential for energy homeostasis
Update in
-
State-dependent central synaptic regulation by GLP-1 is essential for energy homeostasis.Nat Metab. 2025 Jul;7(7):1443-1458. doi: 10.1038/s42255-025-01305-x. Epub 2025 Jun 4. Nat Metab. 2025. PMID: 40467923
Abstract
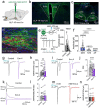
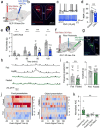
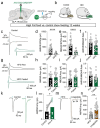
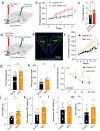
Central nervous system (CNS) control of metabolism plays a pivotal role in maintaining energy homeostasis. Glucagon-like peptide-1 (GLP-1, encoded by Gcg), secreted by a distinct population of neurons located within the nucleus tractus solitarius (NTS), suppresses feeding through projections to multiple brain targets1-3. Although GLP-1 analogs are proven clinically effective in treating type 2 diabetes and obesity4, the mechanisms of GLP-1 action within the brain remain unclear. Here, we investigate the involvement of GLP-1 receptor (GLP-1R) mediated signaling in a descending circuit formed by GLP-1R neurons in the paraventricular hypothalamic nucleus (PVNGLP-1R) that project to dorsal vagal complex (DVC) neurons of the brain stem in mice. PVNGLP- 1R→DVC synapses release glutamate that is augmented by GLP-1 via a presynaptic mechanism. Chemogenetic activation of PVNGLP-1R→DVC neurons suppresses feeding. The PVNGLP-1R→DVC synaptic transmission is dynamically regulated by energy states. In a state of energy deficit, synaptic strength is weaker but is more profoundly augmented by GLP-1R signaling compared to an energy-replete state. In an obese state, the dynamic synaptic strength changes in the PVNGLP-1R→DVC descending circuit are disrupted. Blocking PVNGLP-1R→DVC synaptic release or ablation of GLP-1R in the presynaptic compartment increases food intake and causes obesity, elevated blood glucose, and impaired insulin sensitivity. These findings suggest that the state-dependent synaptic plasticity in this PVNGLP-1R→DVC descending circuit mediated by GLP-1R signaling is an essential regulator of energy homeostasis.
Conflict of interest statement
Conflict of Interests The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

