This is a preprint.
Understanding the Utility of Endocardial Electrocardiographic Imaging in Epi-Endocardial Mapping of 3D Reentrant Circuits
- PMID: 38559058
- PMCID: PMC10980114
- DOI: 10.1101/2024.03.13.24304259
Understanding the Utility of Endocardial Electrocardiographic Imaging in Epi-Endocardial Mapping of 3D Reentrant Circuits
Abstract
Background: Studies of VT mechanisms are largely based on a 2D portrait of reentrant circuits on one surface of the heart. This oversimplifies the 3D circuit that involves the depth of the myocardium. Simultaneous epicardial and endocardial (epi-endo) mapping was shown to facilitate a 3D delineation of VT circuits, which is however difficult via invasive mapping.
Objective: This study investigates the capability of noninvasive epicardial-endocardial electrocardiographic imaging (ECGI) to elucidate the 3D construct of VT circuits, emphasizing the differentiation of epicardial, endocardial, and intramural circuits and to determine the proximity of mid-wall exits to the epicardial or endocardial surfaces.
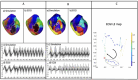
Methods: 120-lead ECGs of VT in combination with subject-specific heart-torso geometry are used to compute unipolar electrograms (CEGM) on ventricular epicardium and endocardia. Activation isochrones are constructed, and the percentage of activation within VT cycle length is calculated on each surface. This classifies VT circuits into 2D (surface only), uniform transmural, nonuniform transmural, and mid-myocardial (focal on surfaces). Furthermore, the endocardial breakthrough time was accurately measured using Laplacian eigenmaps, and by correlating the delay time of the epi-endo breakthroughs, the relative distance of a mid-wall exit to the epicardium or the endocardium surfaces was identified.
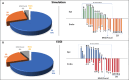
Results: We analyzed 23 simulated and in-vivo VT circuits on post-infarction porcine hearts. In simulated circuits, ECGI classified 21% as 2D and 78% as 3D: 82.6% of these were correctly classified. The relative timing between epicardial and endocardial breakthroughs was correctly captured across all cases. In in-vivo circuits, ECGI classified 25% as 2D and 75% as 3D: in all cases, circuit exits and entrances were consistent with potential critical isthmus delineated from combined LGE-MRI and catheter mapping data.
Conclusions: ECGI epi-endo mapping has the potential for fast delineation of 3D VT circuits, which may augment detailed catheter mapping for VT ablation.
Keywords: 3D Ventricular tachycardia; Electrocardiographic imaging; Endocardial Breakthrough.
Figures











Similar articles
-
Noninvasive epicardial and endocardial electrocardiographic imaging of scar-related ventricular tachycardia.J Electrocardiol. 2016 Nov-Dec;49(6):887-893. doi: 10.1016/j.jelectrocard.2016.07.026. Epub 2016 Jul 28. J Electrocardiol. 2016. PMID: 27968777 Free PMC article.
-
Simultaneous Endocardial and Epicardial Delineation of 3D Reentrant Ventricular Tachycardia.J Am Coll Cardiol. 2020 Mar 3;75(8):884-897. doi: 10.1016/j.jacc.2019.12.044. J Am Coll Cardiol. 2020. PMID: 32130924
-
Non-invasive epicardial and endocardial electrocardiographic imaging for scar-related ventricular tachycardia.Europace. 2018 Sep 1;20(FI2):f263-f272. doi: 10.1093/europace/euy082. Europace. 2018. PMID: 29684187 Free PMC article.
-
Combined Endocardial-Epicardial Versus Endocardial Catheter Ablation Alone for Ventricular Tachycardia in Structural Heart Disease: A Systematic Review and Meta-Analysis.JACC Clin Electrophysiol. 2019 Jan;5(1):13-24. doi: 10.1016/j.jacep.2018.08.010. Epub 2018 Sep 26. JACC Clin Electrophysiol. 2019. PMID: 30678778
-
Endo-epicardial vs endocardial-only catheter ablation of ventricular tachycardia: A meta-analysis.J Cardiovasc Electrophysiol. 2019 Sep;30(9):1537-1548. doi: 10.1111/jce.14013. Epub 2019 Jul 2. J Cardiovasc Electrophysiol. 2019. PMID: 31172632
References
-
- de Chillou C, Lacroix D, Klug D, Magnin-Poull I, Marquie C, Messier M, et al. Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation. 2002;105:726–31. - PubMed
-
- Wissner E, Stevenson WG, Kuck KH. Catheter ablation of ventricular tachycardia in ischaemic and non-ischaemic cardiomyopathy: where are we today? A clinical review. Eur Heart J. 2012. Jun;33(12):1440–50. - PubMed
-
- Tung R, Raiman M, Liao H, Zhan X, Chung FP, Nagel R, et al. Simultaneousendocardial and epicardial delineation of 3d reentrant ventricular tachycardia. J Am Coll Cardiol. 2020;75(8):884–97. - PubMed
-
- Stevenson WG. Current treatment of ventricular arrhythmias: state of the art. Heart Rhythm Off J Heart Rhythm Soc. 2013. Dec;10(12):1919–26. - PubMed
-
- Haissaguerre M, Hocini M, Shah AJ, Derval N, Sacher F, Jais P, et al. Noninvasive Panoramic Mapping of Human Atrial Fibrillation Mechanisms: A Feasibility Report. J Cardiovasc Electrophysiol. 2013;24(6):711–7. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
