This is a preprint.
An engineered human cardiac tissue model reveals contributions of systemic lupus erythematosus autoantibodies to myocardial injury
- PMID: 38559188
- PMCID: PMC10979865
- DOI: 10.1101/2024.03.07.583787
An engineered human cardiac tissue model reveals contributions of systemic lupus erythematosus autoantibodies to myocardial injury
Update in
-
An engineered human cardiac tissue model reveals contributions of systemic lupus erythematosus autoantibodies to myocardial injury.Nat Cardiovasc Res. 2024 Sep;3(9):1123-1139. doi: 10.1038/s44161-024-00525-w. Epub 2024 Aug 15. Nat Cardiovasc Res. 2024. PMID: 39195859 Free PMC article.
Abstract
Systemic lupus erythematosus (SLE) is a highly heterogenous autoimmune disease that affects multiple organs, including the heart. The mechanisms by which myocardial injury develops in SLE, however, remain poorly understood. Here we engineered human cardiac tissues and cultured them with IgG fractions containing autoantibodies from SLE patients with and without myocardial involvement. We observed unique binding patterns of IgG from two patient subgroups: (i) patients with severe myocardial inflammation exhibited enhanced binding to apoptotic cells within cardiac tissues subjected to stress, and (ii) patients with systolic dysfunction exhibited enhanced binding to the surfaces of viable cardiomyocytes. Functional assays and RNA sequencing (RNA-seq) revealed that IgGs from patients with systolic dysfunction exerted direct effects on engineered tissues in the absence of immune cells, altering tissue cellular composition, respiration and calcium handling. Autoantibody target characterization by phage immunoprecipitation sequencing (PhIP-seq) confirmed distinctive IgG profiles between patient subgroups. By coupling IgG profiling with cell surface protein analyses, we identified four pathogenic autoantibody candidates that may directly alter the function of cells within the myocardium. Taken together, these observations provide insights into the cellular processes of myocardial injury in SLE that have the potential to improve patient risk stratification and inform the development of novel therapeutic strategies.
Keywords: Autoantibodies; autoimmune disease; cardiac tissue engineering; myocardial inflammation; systemic lupus erythematosus.
Figures
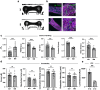

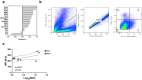
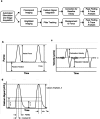
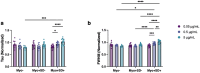
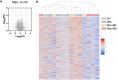
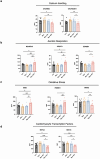

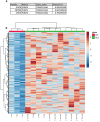
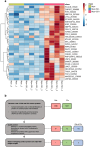
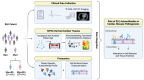
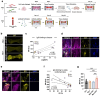
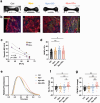
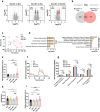

References
-
- Guglin M., Smith C. & Rao R. The spectrum of lupus myocarditis: from asymptomatic forms to cardiogenic shock. Heart Fail Rev 26, 553–560 (2021). - PubMed
-
- Appenzeller S., Pineau C.A. & Clarke A.E. Acute lupus myocarditis: Clinical features and outcome. Lupus 20, 981–988 (2011). - PubMed
-
- Thomas G., et al. Lupus Myocarditis: Initial Presentation and Longterm Outcomes in a Multicentric Series of 29 Patients. J Rheumatol 44, 24–32 (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
