This is a preprint.
A feedback-driven brain organoid platform enables automated maintenance and high-resolution neural activity monitoring
- PMID: 38559212
- PMCID: PMC10979982
- DOI: 10.1101/2024.03.15.585237
A feedback-driven brain organoid platform enables automated maintenance and high-resolution neural activity monitoring
Abstract
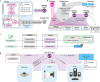
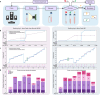
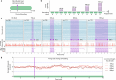
The analysis of tissue cultures, particularly brain organoids, requires a sophisticated integration and coordination of multiple technologies for monitoring and measuring. We have developed an automated research platform enabling independent devices to achieve collaborative objectives for feedback-driven cell culture studies. Our approach enables continuous, communicative, non-invasive interactions within an Internet of Things (IoT) architecture among various sensing and actuation devices, achieving precisely timed control of in vitro biological experiments. The framework integrates microfluidics, electrophysiology, and imaging devices to maintain cerebral cortex organoids while measuring their neuronal activity. The organoids are cultured in custom, 3D-printed chambers affixed to commercial microelectrode arrays. Periodic feeding is achieved using programmable microfluidic pumps. We developed a computer vision fluid volume estimator used as feedback to rectify deviations in microfluidic perfusion during media feeding/aspiration cycles. We validated the system with a set of 7-day studies of mouse cerebral cortex organoids, comparing manual and automated protocols. The automated protocols were validated in maintaining robust neural activity throughout the experiment. The automated system enabled hourly electrophysiology recordings for the 7-day studies. Median neural unit firing rates increased for every sample and dynamic patterns of organoid firing rates were revealed by high-frequency recordings. Surprisingly, feeding did not affect firing rate. Furthermore, performing media exchange during a recording showed no acute effects on firing rate, enabling the use of this automated platform for reagent screening studies.
Keywords: Brain Organoid; Electrophysiology; Internet of Things; Microfluidics; Neural Development; Stem Cells.
Conflict of interest statement
Competing interests K.V. and S.T.S. are co-founders and D.H., S.R.S, M.T. are advisory board members of Open Culture Science, Inc., a company that may be affected by the research reported in the enclosed paper. All other authors declare no competing interests.
Figures



Similar articles
-
A closed 3D printed microfluidic device for automated growth and differentiation of cerebral organoids from single-cell suspension.Biotechnol J. 2024 Aug;19(8):e2400240. doi: 10.1002/biot.202400240. Biotechnol J. 2024. PMID: 39212189
-
Modular automated microfluidic cell culture platform reduces glycolytic stress in cerebral cortex organoids.Sci Rep. 2022 Nov 23;12(1):20173. doi: 10.1038/s41598-022-20096-9. Sci Rep. 2022. PMID: 36418910 Free PMC article.
-
A mesh microelectrode array for non-invasive electrophysiology within neural organoids.Biosens Bioelectron. 2023 May 15;228:115223. doi: 10.1016/j.bios.2023.115223. Epub 2023 Mar 11. Biosens Bioelectron. 2023. PMID: 36931193
-
Emerging Bioelectronics for Brain Organoid Electrophysiology.J Mol Biol. 2022 Feb 15;434(3):167165. doi: 10.1016/j.jmb.2021.167165. Epub 2021 Jul 19. J Mol Biol. 2022. PMID: 34293341 Free PMC article. Review.
-
Microinstrumentation for Brain Organoids.Adv Healthc Mater. 2024 Aug;13(21):e2302456. doi: 10.1002/adhm.202302456. Epub 2024 Jan 26. Adv Healthc Mater. 2024. PMID: 38217546 Review.
References
-
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., Sasai Y.: Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 3(5), 519–532 (2008) 10.1016/j.stem.2008.09.002. Publisher: Elsevier. Accessed 2020-04-14 - DOI - PubMed
-
- Lancaster M.A., Renner M., Martin C.-A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A.: Cerebral organoids model human brain development and microcephaly. Nature 501(7467), 373–379 (2013) 10.1038/nature12517. Number: 7467 Publisher: Nature Publishing Group. Accessed 2021-02-04 - DOI - PMC - PubMed
-
- Pollen A.A., Bhaduri A., Andrews M.G., Nowakowski T.J., Meyerson O.S., Mostajo-Radji M.A., Lullo E.D., Alvarado B., Bedolli M., Dougherty M.L., Fiddes I.T., Kronenberg Z.N., Shuga J., Leyrat A.A., West J.A., Bershteyn M., Lowe C.B., Pavlovic B.J., Salama S.R., Haussler D., Eichler E.E., Kriegstein A.R.: Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 176(4), 743–75617 (2019) 10.1016/j.cell.2019.01.017. Publisher: Elsevier. Accessed 2021-02-03 - DOI - PMC - PubMed
-
- Giandomenico S.L., Mierau S.B., Gibbons G.M., Wenger L.M.D., Masullo L., Sit T., Sutcliffe M., Boulanger J., Tripodi M., Derivery E., Paulsen O., Lakatos A., Lancaster M.A.: Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nature Neuroscience 22(4), 669–679 (2019) 10.1038/s41593-019-0350-2. Accessed 2019-09-29 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
