This is a preprint.
Early intermediates in bacterial RNA polymerase promoter melting visualized by time-resolved cryo-electron microscopy
- PMID: 38559232
- PMCID: PMC10979975
- DOI: 10.1101/2024.03.13.584744
Early intermediates in bacterial RNA polymerase promoter melting visualized by time-resolved cryo-electron microscopy
Update in
-
Early intermediates in bacterial RNA polymerase promoter melting visualized by time-resolved cryo-electron microscopy.Nat Struct Mol Biol. 2024 Nov;31(11):1778-1788. doi: 10.1038/s41594-024-01349-9. Epub 2024 Jul 1. Nat Struct Mol Biol. 2024. PMID: 38951624 Free PMC article.
Abstract
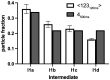
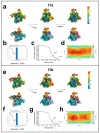
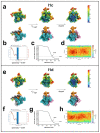
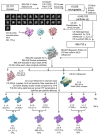
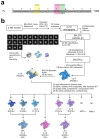
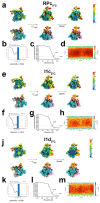
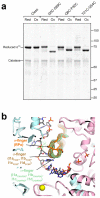
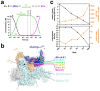
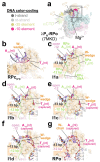
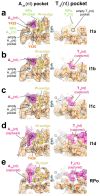
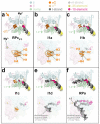
During formation of the transcription-competent open complex (RPo) by bacterial RNA polymerases (RNAP), transient intermediates pile up before overcoming a rate-limiting step. Structural descriptions of these interconversions in real time are unavailable. To address this gap, time-resolved cryo-electron microscopy (cryo-EM) was used to capture four intermediates populated 120 or 500 milliseconds (ms) after mixing Escherichia coli σ70-RNAP and the λPR promoter. Cryo-EM snapshots revealed the upstream edge of the transcription bubble unpairs rapidly, followed by stepwise insertion of two conserved nontemplate strand (nt-strand) bases into RNAP pockets. As nt-strand "read-out" extends, the RNAP clamp closes, expelling an inhibitory σ70 domain from the active-site cleft. The template strand is fully unpaired by 120 ms but remains dynamic, indicating yet unknown conformational changes load it in subsequent steps. Because these events likely describe DNA opening at many bacterial promoters, this study provides needed insights into how DNA sequence regulates steps of RPo formation.
Conflict of interest statement
Competing interests. The authors declare there are no competing interests. The authors declare no conflict of interest
Figures


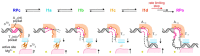
References
-
- Feklistov A., Sharon B. D., Darst S. A. & Gross C. A. Bacterial sigma factors: a historical, structural, and genomic perspective. Annual Review of Microbiology 68, 357–376 (2014). - PubMed
-
- Gruber T. M. & Gross C. A. Multiple sigma subunits and the partitioning of bacterial transcription space. Annual Review of Microbiology 57, 441–466 (2003). - PubMed
-
- Zhang G. et al. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 A resolution. Cell 98, 811–24 (1999). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
