Guideline-directed device therapies in heart failure: A clinical practice-based analysis using electronic health record data
- PMID: 38559281
- PMCID: PMC10976280
- DOI: 10.1016/j.ahjo.2022.100139
Guideline-directed device therapies in heart failure: A clinical practice-based analysis using electronic health record data
Abstract
Background: Guideline-directed device therapies (GDDT) improve outcomes for eligible patients with heart failure (HF) with reduced ejection fraction (HFrEF). Utilization rates of device therapies in HFrEF after the 2012 ACCF/AHA/HRS Focused Update for Device-based Therapies of Cardiac Rhythm Abnormalities have not been well studied.
Objective: Characterize the use of GDDT in newly indicated HFrEF patients from 2012 to 2019 using aggregated electronic health record (EHR) data.
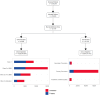
Methods: Computable phenotyping algorithms for implantable cardioverter defibrillator/cardiac resynchronization therapy-defibrillator (ICD/CRT-D) indications from the GuideLine Indications Detected in EHR for Heart Failure program (GLIDE-HF) used diagnoses, procedures, measures, prescriptions, and the output of natural language processed provider notes from de-identified Optum® EHR data. Patients had a diagnosis of HF, dilated cardiomyopathy, or prior infarct, and were included if they had HFrEF with >1 year of records prior to a new Class 1 or Class 2a indication for an ICD or cardiac resynchronization therapy with defibrillator (CRT-D) from 2012 to 2019.
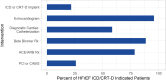
Results: Records showed 137,476 HFrEF patients were newly indicated for an ICD or CRT-D. GDDT was used in 14,892 of 36,358 (41.0%) CRT-D indicated patients and in 14,904 of 101,118 (14.7%) ICD-indicated patients. While GDDT use was low, 95.7% had echocardiography and 92.1% had prescriptions for beta-blockers or angiotensin-converting enzyme/angiotensin-receptor blockers medications.
Conclusions: In this modern cohort of HF patients, a large proportion of eligible patients did not receive ICDs or CRT-Ds, while frequently receiving other indicated cardiovascular interventions and treatments.
Keywords: Cardiac resynchronization therapy; Electronic health record; Guideline-directed device therapy; Heart failure; Implantable cardioverter defibrillator.
© 2022 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CM, LDJ, & DS: Employee/Shareholder – Medtronic, Inc.; ABC: Honoraria/Speaking Fee and data monitoring board for clinical trial – Medtronic, Inc.; advisory board: Janssen Pharmaceuticals, Abbott, Sanofi Aventis, Milestone Pharmaceuticals; honoraria for speaking: Abbott; GCF: Consulting – Abbott, Amgen, CHF Solutions, Janssen, Medtronic, Inc., Merck, and Novartis.
Figures
References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. - PubMed
-
- Meta-analysis Global Group in Chronic Heart F The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur. Heart J. 2012;33(14):1750–1757. - PubMed
-
- Maggioni A.P., Dahlstrom U., Filippatos G., Chioncel O., Crespo Leiro M., Drozdz J., et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur. J. Heart Fail. 2013;15(7):808–817. - PubMed
-
- Epstein A.E., JP DiMarco, Ellenbogen K.A., Estes N.A., III, Freedman R.A., Gettes L.S., et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2013;61(3):e6–e75. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

