Discovery of a Potent Antiosteoporotic Drug Molecular Scaffold Derived from Angelica sinensis and Its Bioinspired Total Synthesis
- PMID: 38559293
- PMCID: PMC10979506
- DOI: 10.1021/acscentsci.3c01414
Discovery of a Potent Antiosteoporotic Drug Molecular Scaffold Derived from Angelica sinensis and Its Bioinspired Total Synthesis
Abstract
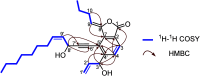
Angelica sinensis, commonly known as Dong Quai in Europe and America and as Dang-gui in China, is a medicinal plant widely utilized for the prevention and treatment of osteoporosis. In this study, we report the discovery of a new category of phthalide from Angelica sinensis, namely falcarinphthalides A and B (1 and 2), which contains two fragments, (3R,8S)-falcarindiol (3) and (Z)-ligustilide (4). Falcarinphthalides A and B (1 and 2) represent two unprecedented carbon skeletons of phthalide in natural products, and their antiosteoporotic activities were evaluated. The structures of 1 and 2, including their absolute configurations, were established using extensive analysis of NMR spectra, chemical derivatization, and ECD/VCD calculations. Based on LC-HR-ESI-MS analysis and DFT calculations, a production mechanism for 1 and 2 involving enzyme-catalyzed Diels-Alder/retro-Diels-Alder reactions was proposed. Falcarinphthalide A (1), the most promising lead compound, exhibits potent in vitro antiosteoporotic activity by inhibiting NF-κB and c-Fos signaling-mediated osteoclastogenesis. Moreover, the bioinspired gram-scale total synthesis of 1, guided by intensive DFT study, has paved the way for further biological investigation. The discovery and gram-scale total synthesis of falcarinphthalide A (1) provide a compelling lead compound and a novel molecular scaffold for treating osteoporosis and other metabolic bone diseases.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
LinkOut - more resources
Full Text Sources
