Preanalytical Errors in Clinical Laboratory Testing at a Glance: Source and Control Measures
- PMID: 38559530
- PMCID: PMC10981510
- DOI: 10.7759/cureus.57243
Preanalytical Errors in Clinical Laboratory Testing at a Glance: Source and Control Measures
Abstract
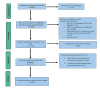
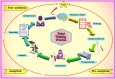
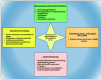
The accuracy of diagnostic results in clinical laboratory testing is paramount for informed healthcare decisions and effective patient care. While the focus has traditionally been on the analytical phase, attention has shifted towards optimizing the preanalytical phase due to its significant contribution to total laboratory errors. This review highlights preanalytical errors, their sources, and control measures to improve the quality of laboratory testing. Blood sample quality is a critical concern, with factors such as hemolysis, lipemia, and icterus leading to erroneous results. Sources of preanalytical errors encompass inappropriate test requests, patient preparation lapses, and errors during sample collection, handling, and transportation. Mitigating these errors includes harmonization efforts, education and training programs, automated methods for sample quality assessment, and quality monitoring. Collaboration between laboratory personnel and healthcare professionals is crucial for implementing and sustaining these measures to enhance the accuracy and reliability of diagnostic results, ultimately improving patient care.
Keywords: biological variation; blood sample quality; hemolysis; laboratory error; laboratory process; laboratory quality; laboratory sample rejection; phelebotomy; pre-analytical variables; results inaccuracy.
Copyright © 2024, Nordin et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Pre-analytical phase in clinical chemistry laboratory. Neogi SS, Mehndiratta M, Gupta S, Puri D. J Clin Sci Res. 2016;5:171–178.
-
- The preanalytical phase - past, present and future. Cornes M. Ann Clin Biochem. 2020;57:4–6. - PubMed
-
- Blood sample quality. Lippi G, von Meyer A, Cadamuro J, Simundic AM. Diagnosis (Berl) 2019;6:25–31. - PubMed
-
- Managing hemolyzed samples in clinical laboratories. Simundic AM, Baird G, Cadamuro J, Costelloe SJ, Lippi G. Crit Rev Clin Lab Sci. 2020;57:1–21. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
