Myocardial Matrix Hydrogels Mitigate Negative Remodeling and Improve Function in Right Heart Failure Model
- PMID: 38559631
- PMCID: PMC10978413
- DOI: 10.1016/j.jacbts.2024.01.006
Myocardial Matrix Hydrogels Mitigate Negative Remodeling and Improve Function in Right Heart Failure Model
Abstract
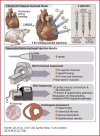
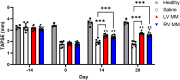
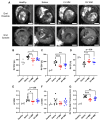
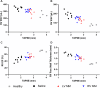
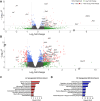
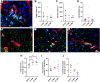
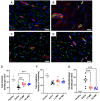
This study evaluates the effectiveness of myocardial matrix (MM) hydrogels in mitigating negative right ventricular (RV) remodeling in a rat model of RV heart failure. The goal was to assess whether a hydrogel derived from either the right or left ventricle could promote cardiac repair. Injured rat right ventricles were injected with either RV-or left ventricular-derived MM hydrogels. Both hydrogels improved RV function and morphology and reduced negative remodeling. This study supports the potential of injectable biomaterial therapies for treating RV heart failure.
Keywords: biomaterial; extracellular matrix; hydrogel; negative right ventricular remodeling; right ventricle.
© 2024 The Authors.
Conflict of interest statement
This research was funded in part by the NIH National Heart, Lung, and Blood Institute (NHLBI) grant R01HL146147 (Drs Davis and Christman). Dr Hunter was supported by an NIH NHLBI Training Grant (T32HL105373) and an NIH NHLBI Predoctoral Fellowship (1F31HL158212). Dr Christman is co-founder, consultant, board member, and holds an equity interest in Ventrix Bio, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Similar articles
-
Characterization of decellularized left and right ventricular myocardial matrix hydrogels and their effects on cardiac progenitor cells.J Mol Cell Cardiol. 2022 Oct;171:45-55. doi: 10.1016/j.yjmcc.2022.06.007. Epub 2022 Jul 1. J Mol Cell Cardiol. 2022. PMID: 35780862 Free PMC article.
-
Cardiac Fibrosis in the Pressure Overloaded Left and Right Ventricle as a Therapeutic Target.Front Cardiovasc Med. 2022 May 6;9:886553. doi: 10.3389/fcvm.2022.886553. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35600469 Free PMC article. Review.
-
The novel P2X7 receptor antagonist PKT100 improves cardiac function and survival in pulmonary hypertension by direct targeting of the right ventricle.Am J Physiol Heart Circ Physiol. 2020 Jul 1;319(1):H183-H191. doi: 10.1152/ajpheart.00580.2019. Epub 2020 May 29. Am J Physiol Heart Circ Physiol. 2020. PMID: 32469637
-
Evaluation of a polyurethane-reinforced hydrogel patch in a rat right ventricle wall replacement model.Acta Biomater. 2020 Jan 1;101:206-218. doi: 10.1016/j.actbio.2019.10.026. Epub 2019 Oct 22. Acta Biomater. 2020. PMID: 31654774 Free PMC article.
-
The role of regenerative therapy in the treatment of right ventricular failure: a literature review.Stem Cell Res Ther. 2020 Nov 25;11(1):502. doi: 10.1186/s13287-020-02022-w. Stem Cell Res Ther. 2020. PMID: 33239066 Free PMC article. Review.
Cited by
-
Myocardial Matrix Hydrogels for Cardiac Repair: The Devil Is in the Details.JACC Basic Transl Sci. 2024 Mar 28;9(3):339-341. doi: 10.1016/j.jacbts.2024.02.001. eCollection 2024 Mar. JACC Basic Transl Sci. 2024. PMID: 38559625 Free PMC article.
-
Protein-Like Polymers Targeting Keap1/Nrf2 as Therapeutics for Myocardial Infarction.Adv Mater. 2025 Jul;37(27):e2417885. doi: 10.1002/adma.202417885. Epub 2025 Apr 25. Adv Mater. 2025. PMID: 40277240 Free PMC article.
References
-
- Voelkel N.F. In: Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease. Friedberg M.K., Redington A.N., editors. Springer Science + Business Media; 2018. How does the pressure-overloaded right ventricle adapt and why does it fail? Macro-and micro-molecular perspectives; pp. 19–27.
Grants and funding
LinkOut - more resources
Full Text Sources

