Reducing Abdominal Aortic Aneurysm Progression by Blocking Neutrophil Extracellular Traps Depends on Thrombus Formation
- PMID: 38559632
- PMCID: PMC10978405
- DOI: 10.1016/j.jacbts.2023.11.003
Reducing Abdominal Aortic Aneurysm Progression by Blocking Neutrophil Extracellular Traps Depends on Thrombus Formation
Abstract
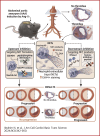
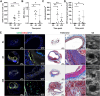
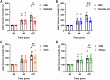
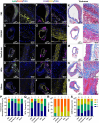
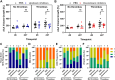
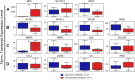
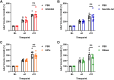
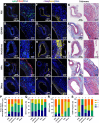
Neutrophil extracellular traps (NETs) are implicated in the pathogenesis of abdominal aortic aneurysm (AAA), located in adventitia and intraluminal thrombus. We compared the therapeutic potential of targeting upstream or downstream effector molecules of NET formation in 2 murine AAA models based on angiotensin II or peri-adventitial elastase application. In both models, NETs were detected in formed aneurysms at treatment start. Although NET inhibitors failed in the elastase model, they prevented progression of angiotensin II-induced aneurysms with thrombus, which resembles established human disease (including thrombus development). Blockade of upstream NET mediators was more effective than interference with downstream NET molecules.
Keywords: abdominal aortic aneurysm; mouse model; neutrophil extracellular trap; thrombus.
© 2024 The Authors.
Conflict of interest statement
This research was funded in whole by the Austrian Science Fund (FWF) [F 5409-B21 issued to Dr Brostjan]. For the purpose of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. Dr Bailey is personally supported by the British Heart Foundation (FS/18/12/33270) and Ms Knöbl by the DocFund program (DOC 59-833) of the Austrian Science Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

