Interrupted SNAr-Alkylation Dearomatization
- PMID: 38559710
- PMCID: PMC10976598
- DOI: 10.1021/jacsau.3c00813
Interrupted SNAr-Alkylation Dearomatization
Abstract
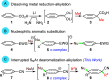
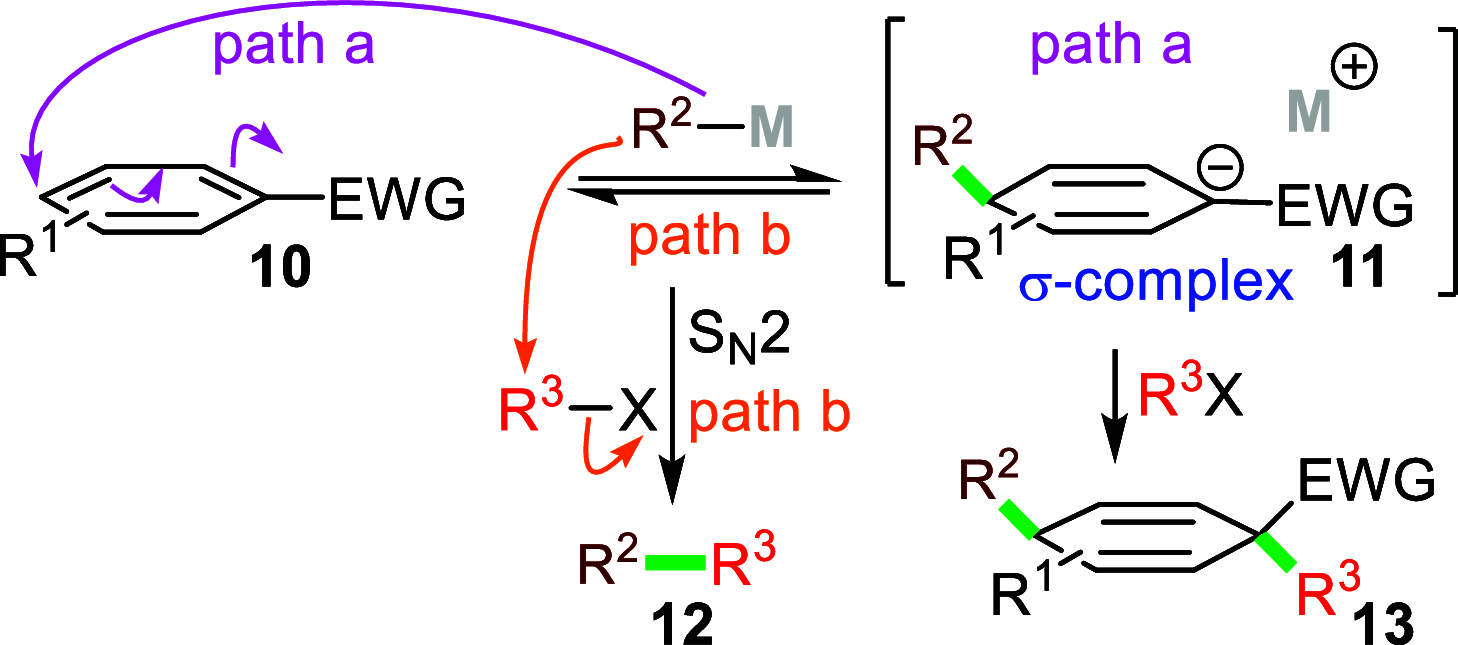
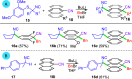
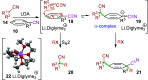
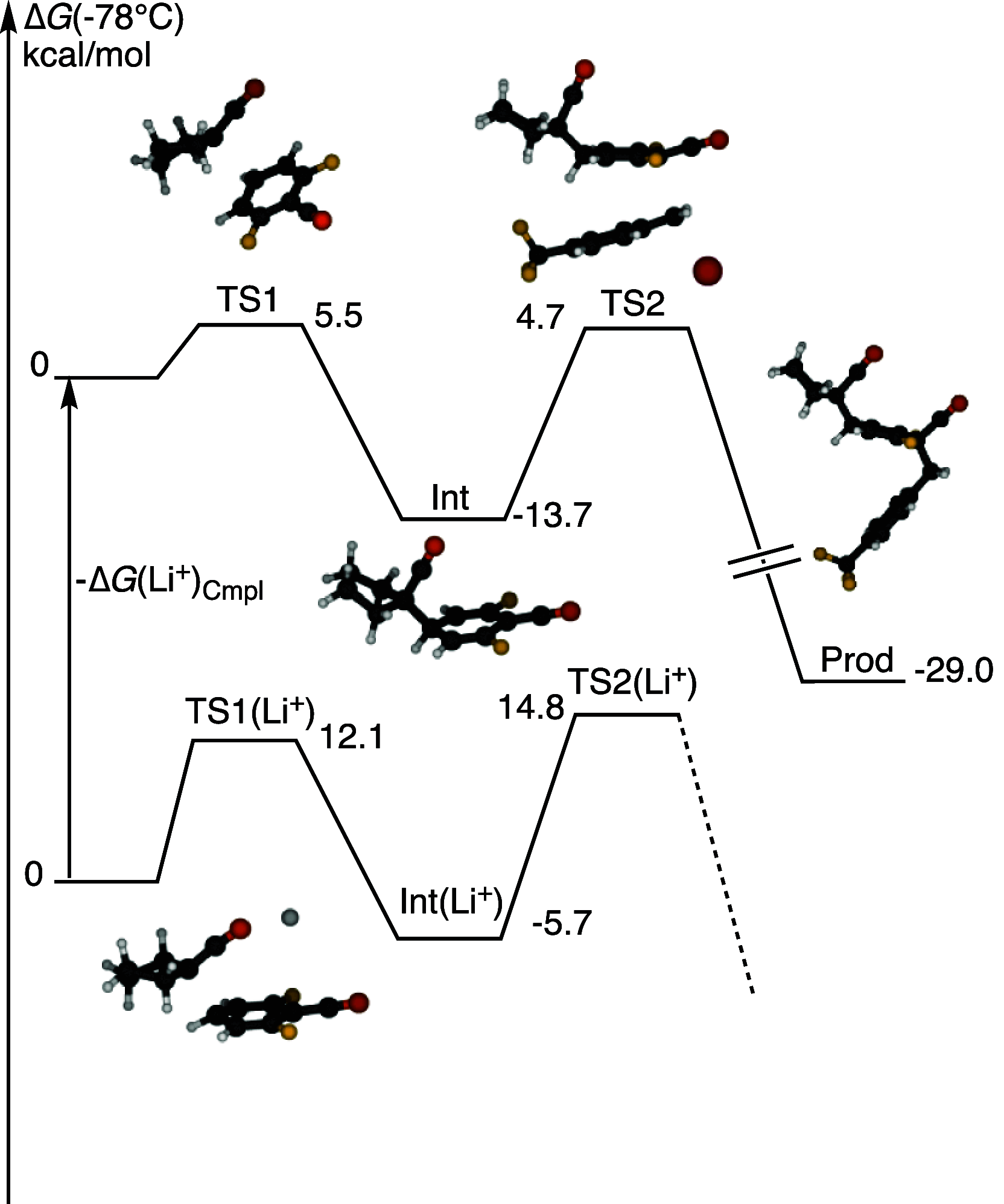
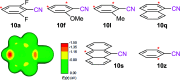
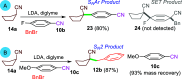
Dearomatizations provide powerful synthetic routes to rapidly assemble substituted carbocycles and heterocycles found in a plethora of bioactive molecules. Harnessing the advantages of dearomatization typically requires vigorous reagents because of the difficulty in disrupting the stable aromatic core. A relatively mild dearomatization strategy is described that employs lithiated nitriles or isocyanides in a simple SNAr-type addition to form σ-complexes that are trapped by alkylation. The dearomatizations are diastereoselective and efficient and rapidly install two new carbon-carbon bonds, one of which is a quaternary center, as well as nitrile, isocyanide, and cyclohexadiene functionalities.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Industrial Arene Chemistry: Markets, Technologies, Sustainable Processes and Cases Studies of Aromatic Commodities; Mortier J., Ed.; Wiley VCH: Weinheim, Germany, 2023.
LinkOut - more resources
Full Text Sources
Research Materials
